24 Jan 2022
4-year outcomes after left atrial appendage closure versus Nonwarfarin oral anticoagulation for atrial fibrillation
Selected in Journal of the American College of Cardiology by A. N. Calik
Left atrial appendage closure (LAAC) is a promising interventional treatment option for preventing cardioembolic events in non-valvular atrial fibrillation (AF) patients who can not tolerate long-term oral anticoagulation.
References
Authors
Pavel Osmancik, Dalibor Herman, Petr Neuzil, Pavel Hala, Milos Taborsky, Petr Kala, Martin Poloczek, Josef Stasek, Ludek Haman, Marian Branny, Jan Chovancik, Pavel Cervinka, Jiri Holy, Tomas Kovarnik, David Zemanek, Stepan Havranek, Vlastimil Vancura, Petr Peichl, Petr Tousek, Veronika Lekesova, Jiri Jarkovsky, Martina Novackova, Klara Benesova, Petr Widimsky, Vivek Y. Reddy, and on behalf of the PRAGUE-17 Trial Investigators
Reference
J Am Coll Cardiol. 2022 Jan, 79 (1) 1–14
Published
January 2022
Link
Read the abstract
Reviewer
My Comment
Why this study – the rationale/objective?
Left atrial appendage closure (LAAC) is a promising interventional treatment option for preventing cardioembolic events in non-valvular atrial fibrillation (AF) patients who can not tolerate long-term oral anticoagulation.
The earlier results of the PRAGUE-17 trial, which compares LAAC with direct oral anticoagulants (DOACs), were elegantly summarized by Salvatore Brugaletta for PCRonline Journal Club. At 20 months, the LAAC was found to be non-inferior to DOACs for the primary composite outcome of stroke, transient ischemic attack (TIA), systemic embolism (SE), cardiovascular death, major or nonmajor clinically relevant bleeding, and procedure-/device-related complications in nonvalvular AF patients.
With an expectation of lower bleeding events in the long-term follow-up, particularly after withdrawing oral anticoagulants and/or antiplatelet therapy (DAPT) in the LAAC group, an extended follow-up for the study population was planned.
How was it executed? - the methodology
The PRAGUE-17 is an investigator-initiated, multicenter, prospective, open-label, randomized, and noninferiority trial.
Non-valvular AF patients with a history of bleeding requiring intervention or hospitalization, history of cardioembolism while taking anticoagulation, or a moderate to high-risk profile, defined as CHA2DS2-VASc ≥ 3 plus HAS-BLED ≥ 2, were included in the study.
Key exclusion criteria include mechanical valve prosthesis, mitral stenosis, comorbidities other than AF mandating anticoagulation.
Patients were randomized to receive LAAC or DOAC. After LAAC, the recommended antithrombotic regimen was aspirin 100 mg/d plus clopidogrel 75 mg/d for 3 months, then aspirin 100 mg/d indefinitely.
Nevertheless, the post-LAAC antithrombotic regimen was left to the physician's discretion to be modified following the patient's characteristics and device type.
The primary outcome was a composite of stroke (ischemic or hemorrhagic) or TIA; systemic embolism; clinically significant bleeding; CV death; or a significant periprocedural or device-related complication.
The primary analysis was prespecified to be performed on a modified intention-to-treat (mITT) basis, including all randomized patients without an LAA thrombus by TEE. Also, post hoc secondary per-protocol and on-treatment analyses were performed.
What is the main result?
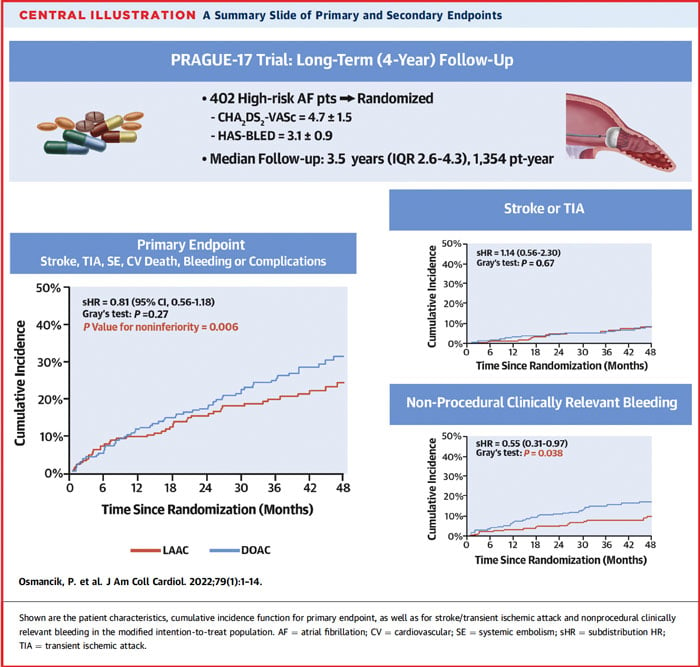
A total of 201 patients were randomized to each group and followed for a median of 3.5 years. The mean age was 73 years, 34.3 % were women, mean CHA2DS2-VASc = 4.7 ±1.5 (> 25 % being CHA2DS2-VASc > 6) and mean HAS-BLED was 3.1 ± 0.9. The implanted devices were Amulet, Watchman, or Watchman-FLX in 61.3 %, 35.9 %, or 2.8 %, respectively. In the DOAC group, the most frequently used anticoagulant was apixaban in 192 patients (95.5 %).
At a median of 3.5 years, the annualized rates of the primary composite outcome were 8.6 % with LAAC and 11.9 % with DOACs (sHR: 0.81; 95 % CI: 0.56-1.18; P = 0.27, P-value for noninferiority = 0.006).
As for the individual components of the primary outcome, the rates of CV death, all-stroke/TIA, systemic embolism, and clinically relevant bleeding were not different between the two groups.
However, the annualized incidence of nonprocedural clinically relevant bleeding was significantly lower in the LAAC group (3.4 % vs 5.9 %, sHR:0.55; 95% CI: 0.31-0.97; P = 0.039).
Consistent findings were also observed in post hoc per protocol and on-treatment analysis.
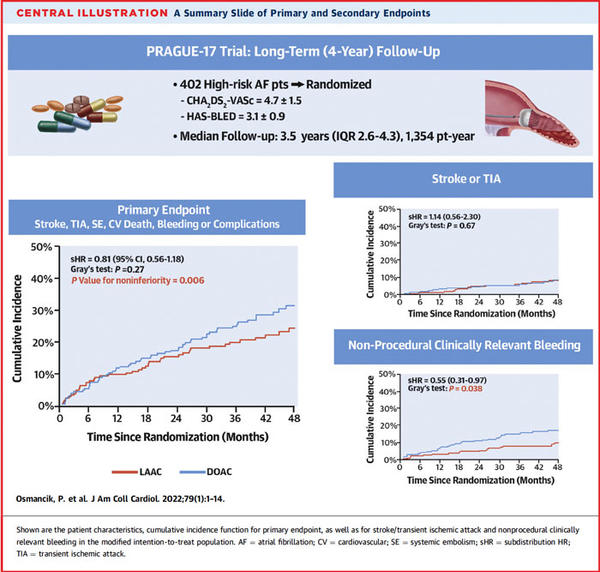
PRAGUE-17 trial: a summary slide of primary and secondary endpoints
Source: Journal of the American College of Cardiology
Critical reading and the relevance for clinical practice
In this extended follow-up analysis of the PRAGUE 17 trial, there were no significant differences in all-stroke/TIA or CV death rates between groups. On the other hand, the incidence of nonprocedural clinically relevant bleeding was significantly reduced with LAAC in all presented analyses. As might be anticipated, this difference was primarily driven by the ceasing antithrombotic therapy over time, which caused the nonprocedural bleeding curves to diverge approximately 6 months after randomization.
While implementing the study results to daily practice, one should keep in mind that the study was not powered to evaluate the relative differences in individual components of the primary composite endpoint. Besides, the rates of device-related thrombus and peridevice leak, which may potentially increase the risk of cardioembolism in the LAAC group, could not be provided due to the COVID-19 pandemic, even intended. Notwithstanding, the incidence of stroke and systemic embolism remained similar in the LAAC and DOAC groups over time.
In a nutshell, the four-year results of the PRAGUE 17 trial highlight the efficacy and safety of LAAC in non-valvular AF patients who can not tolerate or are not willing to receive even DOAC therapy.