Balloon- versus self-expanding valve systems for treating small failed surgical aortic bioprostheses: the LYTEN trial
Selected in JACC by L. Biasco
Luigi provides us with the interventional cardiologist's point of view!
Currently, TAVR selection process is performed by matching the internal diameter of the surgically implanted bioprostheses to the external diameter of commercially available TAVR devices. Nonetheless, the impact of intrinsic design differences, such as supra vs intra-annular design, remains largely unknown.
References
Authors
Josep Rodés-Cabau, Amr Abbas, Vicenç Serra, Victoria Vilalta, Luis Nombela-Franco, Ander Regueiro, Karim M. Al-Azizi, Ayman Iskander, Lenard Conradi, Jessica Forcillo, Scott Lilly, Alvaro Calabuig, Eduard Fernandez-Nofrerias, Siamak Mohammadi, Vassili Panagides, Emilie Pelletier-Beaumont, and Philippe Pibarot
Reference
J Am Coll Cardiol. May 18, 2022. Epublished DOI: 10.1016/j.jacc.2022.05.005
Published
18 May 2022
Link
Read the abstract
Reviewer
Latest contributions
TAVI complications - Part 5 Unusual structural interventions: infective endocarditis, ventricular septal rupture & hypertrophic obstructive cardiomyopathy Tendyne transcatheter mitral valve system in patients with severe mitral annular calcification: one-year outcomes from the SUMMIT Severe MAC cohortMy Comment
Why this study – the rationale/objective?
Treatment of patients with failed aortic bioprostheses is nowadays a common indication for TAVR-in-valve implantation.
In particular, in patients with previously implanted small (≤ 23 mm) surgical valves, selection and sizing of TAVR remain crucial in order to adequately optimize hemodynamics, prevent recurrent structural valve deterioration, and, ideally, decrease the risk of patient-prosthesis mismatch.
Currently, TAVR selection process is performed by matching the internal diameter of the surgically implanted bioprostheses to the external diameter of commercially available TAVR devices. Nonetheless, the impact of intrinsic design differences, such as supra vs intra-annular design, remains largely unknown.
How was it executed? - the methodology
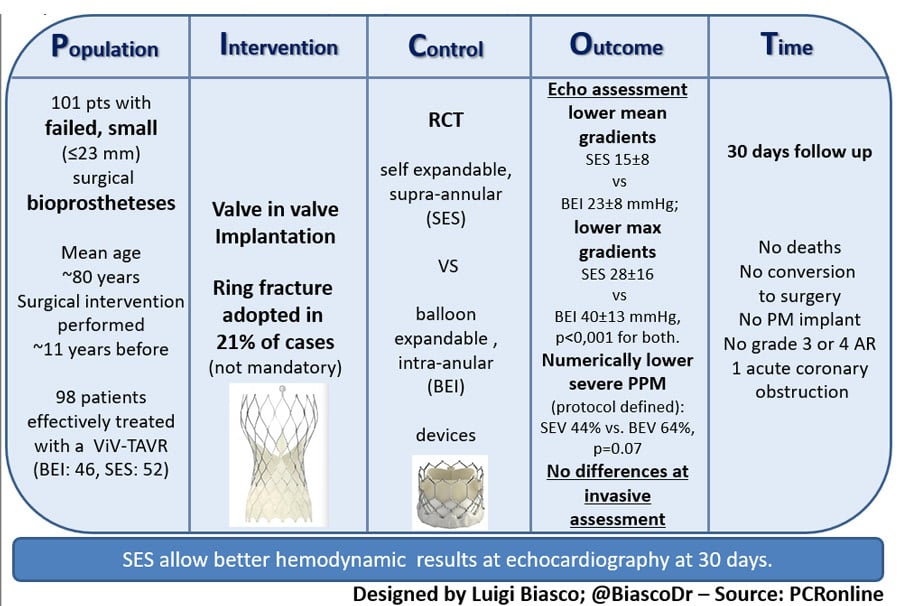
Prospective, investigator-initiated, randomized valve-in-valve trial conducted among 11 Canadian, US, and European centers comparing SAPIEN 3 / ULTRA valve (balloon-expandable valve with intra-annular leaflet design) to the Evolut R /PRO / PRO+ (self-expandable with supra-annular leaftlet design).
Study population:
- 102 patients with failed small (≤ 23 mm and ≤ 21 mm inner diameter) stented surgical bioprosthesis randomized between May 2017 and January 2022. Patients with valves with externally mounted leaflets were also included.
- Patients with stentless or sutureless surgical valve were excluded from the study.
Methodology:
- Randomization to a 20-23 mm balloon-expandable (BEV) device with intra-annular leaflets SAPIEN 3 / ULTRA valve or a self-expandable (SEV) supra-annular 23-26 mm Evolut R / PRO / PRO+ valve --> 98 patients effectively treated with a ViV-TAVR procedure (BEV: 46, SEV: 52)
- Surgical ring fracture was not recommended per protocol but left at discretion of heart team decision.
- Hemodynamic performance of the valve at 30 days was set as the primary endpoint:
- Maximal / mean residual gradients were assessed at echocardiography;
- Presence of severe prosthesis patient mismatch (PPM) was assessed. This was defined as an indexed effective orifice area (EOA) ≤ 0.65 cm2/m2 (per protocol definition) and according to the VARC-3 definition as indexed EOA ≤ 0.65 cm2/m2 for patients with BMI < 30 Kg/m2 and as an indexed EOA ≤ 0.55 cm2/m2 for patients with BMI ≥ 30 kg/m2.
- Presence of moderate-severe aortic regurgitation. Echocardiographic images were evaluated by a central independent echo core lab. Clinical events were not adjudicated by a clinical event committee.
- Secondary outcomes included clinical endpoints along with invasive hemodynamic measurements at the time of TAVR procedure (the latter was included only after study protocol amendment).
- Due to pandemic-related restrictions, echocardiographic evaluation at 30 days was available in 79 patients (36 BEV and 34 SEV).
What is the main result?
- Mean age of patients was ~ 80 and time from initial surgery was ~ 11 years in both groups.
- Surgical prostheses treated were 40 CE-Perimount Magna Ease (Edwards); 24 Mitroflow (Sorin); 15 Trifecta (Abbott); 4 Mosaic (Medtronic), 3 Hancock II (Medtronic); 2 Epic (Abbott), and 1 each for Dokimos Plus (Labcor) and Avalus (Medtronic).
- Lower trans-prosthetic gradients were found at 30 days in the SEV group as compared to the BEV group (mean gradient: 15 ± 8 vs 23 ± 8 mmHg; max gradient 28 ± 16 and 40 ± 13 mmHg, p < 0,001 for both) when assessed at echocardiography.
- When randomized to BEV, smaller TAVR prostheses were selected.
- Rate of patients with residual gradient > 20 mmHg was higher in the BEV group (62 vs 21 %, p < 0,001) while a non-significant trend towards a higher rate of severe PPM was reported for both definitions used (64 % vs. 44 % p = 0.07 per protocol; 39 % vs. 20 %, p = 0.053, VARC-3 definition).
- No cases of moderate or severe AR were recorded at 30 D.
- 55 consecutive patients were evaluated with invasive hemodynamics (28 BEV and 27 SEV). Mean transvalvular gradient measured at procedure completion did not differ between the groups (BEV: 15±13 mmHg, SEV: 12±9 mmHg, p = 0.41). Invasively measured gradients were significantly lower than those estimated by simultaneous echocardiography or at exams performed at hospital discharge (median delay between invasive assessment and echocardiography of 1 day).
- Ring fracture was performed in 21% of patients. The need for ring fracture was higher in the BEV group (35 % vs. 12 %). Decision to proceed with fracture was mostly taken after TAVR implantation based on the presence of high residual intra-procedural gradients. Ring fracture resulted in a mean reduction of transvalvular gradient of 11 mmHg.
- Successful valve implantation was achieved in all patients with no procedural deaths nor conversion to surgery. One case of acute coronary occlusion occurred, successfully treated. Rate of post procedural PM implantation was 0%. No deaths were recorded at 30-day follow-up.
Source : courtesy of Luigi Biasco
Critical reading and the relevance for clinical practice
Proper selection among different TAVR devices remains challenging when treating patients with failed implanted bioprostheses, in particular when facing patients with small surgical valves.
In clinical practice, this process has to take into account several different aspects that finally concur to the selection of BEV vs SES.
Firstly, the dimensions of the implanted prostheses and their relationships with the surrounding structures such as the sinuses of Valsalva, the sino-tubular junction, and coronary ostia have to be considered. All those structures might show altered anatomies and spatial relationships after surgical aortic valve replacement. Those aspects have to be taken into account in order to verify candidacy and assess the risk of coronary obstruction and plan beforehand a bailout strategy.
In addition, when treating small failed surgical bioprostheses, valve-in-valve implantation represents an additional challenge. In fact, the procedure aspires to correct a failed aortic bioprostheses with percutaneous devices forced by the physical constraints represented by surgical valve. This aspect is of particular relevance when aiming at treating patients with PPM.
Finally, several other additional factors such as vascular access size and operator’s preferences do impact the choice.
This small, multicentre randomized trial comparing BEV vs SEV while confirming feasibility and safety of this approach showed improved hemodynamic with supra-annular self-expandable devices as compared to balloon-expandable valves when assessed at echocardiography (but not at invasive hemodynamic).
Differences between gradients obtained invasively vs echo derived measures were partially justified by technical aspects intrinsic to the Bernoulli simplification and the impact of pressure recovery that might increase doppler derived gradients while impact only marginally on invasive measures.
Due to the small sample size and the short follow-up duration, no inference can be made on the possibility that the difference in mean gradient observed at echocardiography (but not at invasive hemodynamic) might translate into a mid and long-term benefit. Confirmation of these observations in a larger population is thus needed.
Nonetheless, this work adds a piece of knowledge suggesting that supra-annular, self-expandable devices might allow better echocardiographic results in this challenging context.






No comments yet!