Ejection Fraction Improvement Following Contemporary High-Risk Percutaneous Coronary Intervention: RESTORE EF Study Results
Selected in Journal of the Society for Cardiovascular Angiography & Interventions by M. Alasnag , U. N. Ibebuogu
Wollmuth et al in the RESTORE EF study sought to examine the impact of Impella assisted HRPCI on LV function at 90-day follow-up.
References
Authors
JasonWollmuth, Mitul P.Patel, Thom Dahle, Aditya Bharadwaj, Thomas E.Waggoner, Jeffrey W.Chambers, Ernesto Ruiz-Rodriguez, Ehtisham Mahmud, Craig Thompson, Lynn Morris, RESTORE EF Investigators
Published
13 August 2022
Link
https://www.sciencedirect.com/science/article/pii/S2772930322003349Reviewers
Our Comment
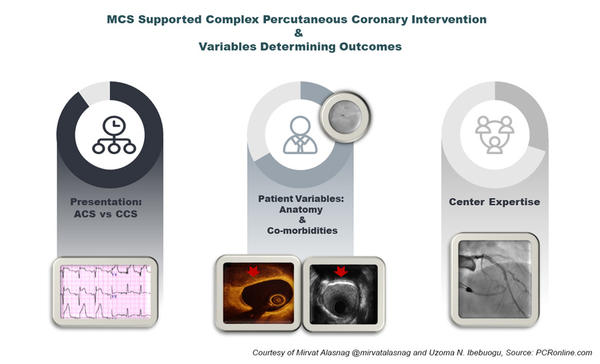
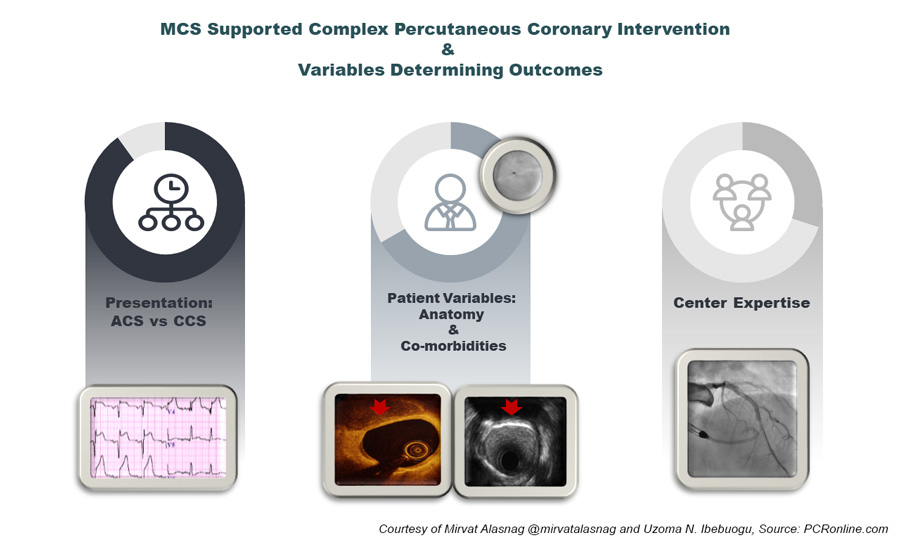
MCS Supported Complex PCI and Variables Determining Outcomes - figure courtesy of Mirvat Alasnag and Uzoma N. Ibebuogu
Why this study – the rationale/objective?
With the ever-expanding scrutiny on outcomes following surgical and percutaneous interventions, several patients with complex multivessel coronary artery disease and co-existing comorbidities are increasingly being turned down for coronary artery bypass graft surgery. Some of the surgical turn down patients end up undergoing high-risk percutaneous coronary intervention (HRPCI) using the Food and Drug Administration (FDA) approved Impella percutaneous left ventricular assist device. The FDA approval of the Impella device for HRPCI was informed by the PROTECT II trial, which had left ventricular ejection fraction (LVEF) <35% as one of the trial inclusion criteria, however, little data exists on the subsequent impact if any on the LVEF following HRPCI with prophylactic Impella support.1
Given the lack of available data to inform clinicians on this important outcome metric, Wollmuth et al in the RESTORE EF study sought to examine the impact of Impella assisted HRPCI on LV function at 90-day follow-up.
How was it executed – the methodology?
The study is a multicenter, retrospective analysis of prospectively collected observational data. The study cohort included patients who underwent elective or urgent Impella assisted HRPCI with specified inclusion and exclusion criteria. The study cohort received either the Impella 2.5 or CP device.
The primary study endpoint was LVEF at 90 days following HRPCI. The secondary endpoints included change in heart failure and angina symptoms at follow up and completeness of revascularization based on pre and post procedure SYNTAX scores.
Paired t test was used to compare baseline and 90-day LVEF measurements. Categorical and continuous data were compared using χ2 analysis and unpaired t test respectively. A P value < .05 was considered significant.
What is the main result?
A total of 406 patients were enrolled from 22 sites in the United States treated between August 2019 to May 2021 of whom 26% were women. The mean age was 70.2 ± 11.4 years. Co-morbidities were not described; however, 77% were reported as surgical turndowns with a SYNTAX score II of 52.6 ± 15.0. The baseline Left Ventricular Ejection Fraction was 37 ± 16%. 62% were classified as NYHA class III or IV at baseline assessment (32% class IV, 30% class III) and 72% reported CCS class III or IV at baseline assessment (44% class IV, 28% class III). Viability was performed in only 11.7% of the population.
In terms of procedural details, an urgent procedure was necessary in only 26.8% of the cases and an Impella CP was utilized in 73.2% with a mean duration of support of 3.9 hours. An average of 2.9 ± 1.1 lesions were treated. 52.3% were treated with atherectomy and vascular complications requiring blood transfusion occurred in 2.5% of patients.
At follow-up, a significant number of patients reported an improvement in their NYHA class with class III/IV decreasing to 15% (P < .0001) and CCS Angina classification decreasing to 2%. The readmission rate was 10.5% and survival to the conclusion of the study 186 days was 97.3%. Interestingly, there was a significant improvement in LVEF from 35 ± 15% to 45 ± 14% at 90 days (P < .0001).
Critical reading and relevance for clinical practice
The use of mechanical circulatory support for high-risk percutaneous coronary interventions has increased in the last decade particularly as the technology evolved with advancements in the level of support, single access, and peel away sheath. However, the evidence capturing practice patterns and reporting improvements in meaningful quality of life endpoints or benefit are grossly lacking. Furthermore, improvements in the LVEF have not been demonstrated in most registries and studies evaluating the role of MCS in patients with an impaired LV systolic function. The RESTORE EF noted a remarkable improvement from 35 ± 15% to 45 ± 14% not previously reported. It should be noted, however, that patients in whom complete revascularization was achieved with a residual SYNTAX score of 0 extracted the most benefit with significant improvement in LVEF at 90 days. There could be a selection bias here as these are all patients in who survived to the follow up period and of course had anatomy that was suitable for a complete revascularization. Data from the British Cardiovascular Intervention Society Database reported worse outcomes in those with PCI of the last remaining vessel (LRV). Mortality rates for those with LRV compared with all vessels was 12 % versus 1.5 %, P<0.001), at 30 days (15% versus 2%, P<0.001) and at one-year (24% versus 5%, P<0.001). Even after adjustment for differences in baseline clinical and procedural characteristics, clinical outcomes remained worse with the last remaining vessel where complete revascularization was not feasible.2
The impact of residual disease defined by the residual SYNTAX score has been ascertained in other PCI trials most notably the Outcomes of Surgically Ineligible Patients With Multivessel CAD (OPTIMUM) registry.3
In this trial, the baseline SYNTAX score was 32.4 and the residual score was 15. In a sub-analysis of those with a residual score <8, more complete revascularization was achieved, and the reported 30-day mortality rate was lower albeit statistically insignificant (3.9% vs 6.3%; P = .19). In the RESTORE EF, the baseline SYNTAX score was 29.7 and the residual SYNTAX score was 5.4 suggesting a lower risk population. The PROTECT III study demonstrated more completeness of revascularization, less bleeding, and improved 90-day clinical outcomes compared to a historic population from the PROTECT II study following for Impella-supported high-risk PCI among patients with severely depressed LVEF (MACCE was 15.1% and 21.9% in PROTECT III and PROTECT II, respectively (p=0.037)). In addition, there was greater improvement achieved in myocardial ischemia jeopardy scores (7.0±2.4 vs 4.4±2.9; P < .001) and SYNTAX scores (21.4±10.8 vs 15.7±9.5; P < .001) in PROTECT III compared with PROTECT II. This suggests that experience with MCS in terms of patient selection and device management has improved over the last decade yielding better outcomes.4
What is also interesting in this study is that viability testing was only required in 11.7% of the cohort suggesting that the prognostic role of imaging prior to revascularization is not essential which is aligned with some historic studies like the Surgical Treatment for Ischemic Heart Failure (STICH) in which myocardial viability was not associated with a long-term benefit following surgical revascularization. Viable myocardium was associated with an overall improvement in left ventricular systolic function that did not translate into a long-term survival.5
Although the data presented by Wollmuth et al is promising, there are variables that mandate further consideration in a randomized trial, namely, the impact of co-morbidities that render this population inoperable, complex PCI including those requiring atherectomy, left main or bifurcation stenting for complete revascularization and finally the impact of mechanical circulatory support itself. Several ongoing trials will hopefully address these variables including the PROTECT IV and REVIVED BCIS 2 studies .Historically, PROTECT II trial in high-risk patients undergoing PCI indicate that the use of the Impella 2.5L percutaneous LVAD had similar adverse events to IABP at 30 and 90 days.6
The BCIS-1 trial showed no difference in outcomes with mandatory IABP compared with provisional IABP use in similar high-risk patients undergoing PCI.7 The totality of evidence on the use of MCS in select high-risk patients, revealed mortality and reinfarction rates that are comparable to high-risk patients undergoing surgical revascularization such as in the STICH trial. As such, high risk supported PCI is a reasonable option for those who are deemed inoperable at this time. We are still awaiting results of randomized controlled studies that inform guidelines.
References:
- George D. Dangas MD, PhD, Annapoorna S. Kini MD, Samin K. Sharma MD, Jose P.S. Henriques MD, PhD, Bimmer E. Claessen MD, PhD, Simon R. Dixon MBChB, Joseph M. Massaro PhD, Igor Palacios MD, Jeffrey J. Popma MD, E. Magnus Ohman MD, Gregg W. Stone MD, William W. O'Neill MD. Impact of Hemodynamic Support With Impella 2.5 Versus Intra-Aortic Balloon Pump on Prognostically Important Clinical Outcomes in Patients Undergoing High-Risk Percutaneous Coronary Intervention (from the PROTECT II Randomized Trial). Am J Cardiol 2014 Jan;113 (2): 222-228.
- Shoaib A, Rashid M, Kontopantelis E, Sharp A, Fahy EF, Nolan J, Townend J, Ludman P, Ratib K, Azam ZA, Ahmad A, McEntegart M, Mohamed M, Kinnaird T, Mamas MA; British Cardiovascular Intervention Society (BCIS) and the National Institute for Cardiovascular Outcomes Research (NICOR). Clinical Characteristics and Outcomes From Percutaneous Coronary Intervention of Last Remaining Coronary Artery: An Analysis From the British Cardiovascular Intervention Society Database. Circ Cardiovasc Interv. 2020 Sep;13(9):e009049. doi: 10.1161/CIRCINTERVENTIONS.120.009049. Epub 2020 Sep 2.
- Salisbury AC, Kirtane AJ, Ali ZA, Grantham JA, Lombardi WL, Yeh RW, Genereux P, Allen KB, Brown WM, Nugent K, Gosch KL, Karmpaliotis D, Spertus JA, Kandzari DE; OPTIMUM Study Investigators. The Outcomes of Percutaneous RevascularizaTIon for Management of SUrgically Ineligible Patients With Multivessel or Left Main Coronary Artery Disease (OPTIMUM) Registry: Rationale and Design. Cardiovasc Revasc Med. 2022 Aug;41:83-91. doi: 10.1016/j.carrev.2022.01.008. Epub 2022 Jan 31.
- O'Neill WW, Anderson M, Burkhoff D, Grines CL, Kapur NK, Lansky AJ, Mannino S, McCabe JM, Alaswad K, Daggubati R, Wohns D, Meraj PM, Pinto DS, Popma JJ, Moses JW, Schreiber TL, Magnus Ohman E. Improved outcomes in patients with severely depressed LVEF undergoing percutaneous coronary intervention with contemporary practices. Am Heart J. 2022 Jun;248:139-149. doi: 10.1016/j.ahj.2022.02.006. Epub 2022 Feb 19.
- Panza JA, Ellis AM, Al-Khalidi HR, Holly TA, Berman DS, Oh JK, Pohost GM, Sopko G, Chrzanowski L, Mark DB, Kukulski T, Favaloro LE, Maurer G, Farsky PS, Tan RS, Asch FM, Velazquez EJ, Rouleau JL, Lee KL, Bonow RO. Myocardial Viability and Long-Term Outcomes in Ischemic Cardiomyopathy. N Engl J Med. 2019 Aug 22;381(8):739-748. doi: 10.1056/NEJMoa1807365.
- O'Neill WW, Kleiman NS, Moses J, Henriques JP, Dixon S, Massaro J, Palacios I, Maini B, Mulukutla S, Dzavík V, Popma J, Douglas PS, Ohman M. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012 Oct 2;126(14):1717-27. doi: 10.1161/CIRCULATIONAHA.112.098194. Epub 2012 Aug 30.
- Perera D, Stables R, Clayton T, De Silva K, Lumley M, Clack L, Thomas M, Redwood S; BCIS-1 Investigators. Long-term mortality data from the balloon pump-assisted coronary intervention study (BCIS-1): a randomized, controlled trial of elective balloon counterpulsation during high-risk percutaneous coronary intervention. Circulation. 2013 Jan 15;127(2):207-12. doi: 10.1161/CIRCULATIONAHA.112.132209. Epub 2012 Dec 6.





No comments yet!