Evaluating the Use of RenalGuard to Protect Patients at High Risk of AKI - STRENGTH Study
Selected in JACC: Cardiovascular Interventions by N. Ryan
The STRENGTH study is a multicentre prospective randomised trial investigating whether RenalGuard is superior to standard intravenous hydration in preventing contrast induced nephropathy in patients with chronic kidney disease (CKD) undergoing complex cardiovascular procedures.
References
Authors
Sarah Mauler-Wittwer , Horst Sievert , Anna-Maria Ioppolo , Felix Mahfoud , Didier Carrié , Janusz Lipiecki , Georg Nickenig , Jean Fajadet , Siegfried Eckert , Marie-Claude Morice , and Philippe Garot
Published
27 July 2022
Link
https://www.jacc.org/doi/10.1016/j.jcin.2022.05.036Reviewer
Latest contributions
Balloon lithotripsy added to conventional preparation before stent implantation in severely calcified coronary lesions Impact of pullback pressure gradient on clinical outcomes after percutaneous coronary interventions Validation of intravascular ultrasound-defined optimal stent expansion criteria for favorable 1-year clinical outcomesMy Comment
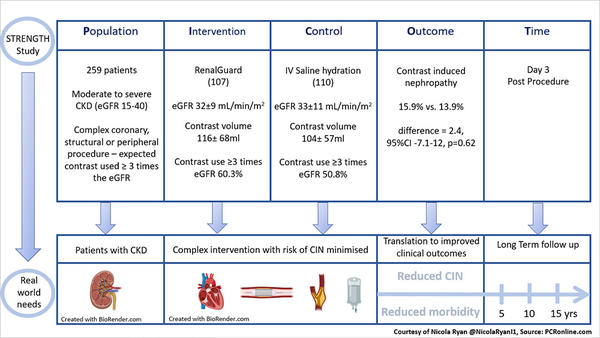
PICOT analysis of the STRENGTH Study - courtesy of Nicola Ryan
Why this study – the rationale/objective?
Contrast induced nephropathy (CIN) is a recognised complication of angiographic procedures, due to the intravascular administration of iodinated contrast media. CIN is an absolute (≥0.33mg/dL, ≥25µmol/L) or relative (≥25%) increase in serum creatinine 48-72 hours post procedure. Baseline patient characteristics as well as procedural complexity, particularly volume of contrast media are risk factors for the development of CIN. To date, no renoprotective strategy has shown clear benefit in prevention of CIN, with IV hydration and minimisation of contrast the current preventative strategies. The RenalGuard system allows hydration and contrast clearance by delivering real-time isotonic IV hydration with matched frusemide induced diuresis whilst maintaining euvolaemia. To date trials of the RenalGuard system have shown mixed results (1–5). The STRENGTH study aimed to assess the superiority of the RenalGuard system over IV hydration in preventing contrast induced nephropathy in patients with chronic kidney disease undergoing complex cardiovascular procedures.
How was it executed – the methodology?
Patients with moderate to severe CKD (eGFR 15-40ml/min/m2) scheduled to undergo a complex coronary, peripheral or structural intervention requiring an estimated contrast dose ≥3 times the eGFR were eligible for inclusion. Participants were randomised 1:1 to RenalGuard therapy (Prehydration with 100-250ml saline over 30 minutes, 0.5mg/kg bolus of frusemide to achieve an optimal urine flow >450ml/h during the procedure and >300ml/h in the postprocedural phase, upto four hours post the last dose of contrast) or treatment according to local practice including oral and IV hydration pre and post procedure, minimisation of contrast and staged procedures as necessary.
- The primary endpoint was the occurrence of CIN, an increase in sCr ≥0.3mg/dL and/or and increase of 25% of basal value at around day 3 or the requirement for dialysis within 5 days after the procedure.
- Secondary endpoints included
- A change in sCr value and eGFR at 12±1 months.
- The percentage of patients on chronic or temporary haemodialysis at 12±1 months.
- A composite of MACCE (cardiac or all-cause death, fatal or non-fatal MI, stroke, target or non-target vessel/lesion revascularisation after the staged procedure).
What is the main result?
Overall, between May 2016 and January 2019, 259 patients (129 RenalGuard, 130 control) were enrolled in the study. Approximately 50% of the population were female with a mean age of 79 years. Baseline mean eGFR was 32ml/min/m2 with 48% of patients undergoing PCI, 18% peripheral interventions and 34% structural. There were no differences in the total volume of contrast used between the groups (RenalGuard 116±68ml vs control 104±57ml, p=0.26). Staged procedures were carried out in 16 patients in the RenalGuard arm and 8 in the control arm.
- There was no difference in CIN between the RenalGuard 17/107 (15.9%) and control 15/110 (13.9%) groups, (difference = 2.4, 95%CI -7.1-12, p=0.62).
- At 12 months the change in sCr value [RG -0.1 (-0.34-0.17) vs control -0.03 (-0.31-0.26), difference -0.05 (-0.19-0.07), p=0.37] and eGFR [RG 3 (-2-8) vs control 1 (-1-4), difference 1(-1-4), p=0.36] did not vary between groups.
- Overall one fifth of patients experienced MACCE at one year with no differences between groups (RG 20.5% vs control 22.2%, p=0.74) or in the components of MACCE.
- Rates of minor bleeding (RG 4.1% vs control 2.4%, p=0.50) and infection (RG 2.4% vs. control 1.6%, p=0.68) related to the urinary catheter insertion were low and did not differ between groups.
Critical reading and relevance for clinical practice
The results of this study show that the use of the RenalGuard system was not superior to standard IV hydration in patients with chronic kidney disease undergoing complex cardiovascular procedures. These results are at odds with a meta-analysis of previous RCTs in patients undergoing cardiovascular procedures with RenalGuard (6). Importantly, the STRENGTH study included higher risk patients with more severe renal impairment as well as complex interventions, situations where CIN may be more likely.
An important difference between this and prior studies is the lower volume of contrast administered during the procedures supporting the evidence that minimisation of contrast is key in preventing CIN. Previous studies have suggested that the risk of renal impairment was reduced by limiting the contrast volume to 3*eGFR (7,8). Despite only including patients undergoing complex procedures with an estimate contrast volume use of 3 times the eGFR contrast volumes in the study were low with only slightly over half the population receiving a contrast volume of >3 times their eGFR (RenalGuard 60.3% vs Control 50.8%). Procedures were carried out high volume interventional centres the volumes of contrast administered in this study compared to prior studies suggests that operators may have already adapted their practice to minimise contrast use. It would have been of interest to understand if additional technology such as the use of IVUS or fusion imaging with prior CT scans was used in this study.
The rates of hydration in the control group was liberal with an intake of 5,029±2,609ml in the 72 hours surrounding the procedure (24 hours pre, day of and 24 hours post). Whilst there is no good evidence surrounding optimal hydration, avoidance of dehydration appears to be key. Although, the rate of complications related to urinary catheters were low given the lack of benefit from the RenalGuard avoidance of urinary catheters and the associated risks would appear prudent.
Overall, the STRENGTH study demonstrates that the RenalGuard system does not reduced the incidence of CIN compared to standard IV hydration in patients with chronic kidney disease undergoing complex coronary interventions. Whilst an optimal protocol for the reduction in CIN is yet to be defined it would appear that minimisation of contrast administered is key. Operators should consider use of all adjunct tools i.e. IVUS, CT fusion which may help reduce contrast volumes. Maintenance of adequate hydration both pre, peri and post procedure may also aid in the reduction of CIN.
References:
- Arbel Y, Ben-Assa E, Puzhevsky D, Litmanowicz B, Galli N, Chorin E, et al. Forced diuresis with matched hydration during transcatheter aortic valve implantation for Reducing Acute Kidney Injury: a randomized, sham-controlled study (REDUCE-AKI). European Heart Journal. 2019 Oct 7;40(38):3169–78.
- Barbanti M, Gulino S, Capranzano P, Immè S, Sgroi C, Tamburino C, et al. Acute Kidney Injury With the RenalGuard System in Patients Undergoing Transcatheter Aortic Valve Replacement: The PROTECT-TAVI Trial (PROphylactic effecT of furosEmide-induCed diuresis with matched isotonic intravenous hydraTion in Transcatheter Aortic Valve Implantation). JACC: Cardiovascular Interventions. 2015 Oct 1;8(12):1595–604.
- Marenzi G, Ferrari C, Marana I, Assanelli E, De Metrio M, Teruzzi G, et al. Prevention of Contrast Nephropathy by Furosemide With Matched Hydration: The MYTHOS (Induced Diuresis With Matched Hydration Compared to Standard Hydration for Contrast Induced Nephropathy Prevention) Trial. JACC: Cardiovascular Interventions. 2012 Jan 1;5(1):90–7.
- Briguori C, Visconti G, Focaccio A, Airoldi F, Valgimigli M, Sangiorgi GM, et al. Renal Insufficiency After Contrast Media Administration Trial II (REMEDIAL II): RenalGuard System in high-risk patients for contrast-induced acute kidney injury. Circulation. 2011 Sep 13;124(11):1260–9.
- Usmiani T, Andreis A, Budano C, Sbarra P, Andriani M, Garrone P, et al. AKIGUARD (Acute Kidney Injury GUARding Device) trial: in-hospital and one-year outcomes. J Cardiovasc Med (Hagerstown). 2016 Jul;17(7):530–7.
- Putzu A, Boscolo Berto M, Belletti A, Pasotti E, Cassina T, Moccetti T, et al. Prevention of Contrast-Induced Acute Kidney Injury by Furosemide With Matched Hydration in Patients Undergoing Interventional Procedures: A Systematic Review and Meta-Analysis of Randomized Trials. JACC: Cardiovascular Interventions. 2017 Feb 27;10(4):355–63.
- Grossman PM, Ali SS, Aronow HD, Boros M, Nypaver TJ, Schreiber TL, et al. Contrast-induced nephropathy in patients undergoing endovascular peripheral vascular intervention: Incidence, risk factors, and outcomes as observed in the Blue Cross Blue Shield of Michigan Cardiovascular Consortium. J Interv Cardiol. 2017 Jun;30(3):274–80.
- Gualano SK, Seth M, Gurm HS, Sukul D, Chetcuti SJ, Patel HJ, et al. Renal Function–Based Contrast Threshold Predicts Kidney Injury in Transcatheter Aortic Valve Replacement. Journal of the Society for Cardiovascular Angiography & Interventions. 2022 May 1;1(3):100038.
:




No comments yet!