A large, prospective, multicentre study of left main PCI using a latest-generation zotarolimus-eluting stent: the ROLEX study
Selected in EuroIntervention by M. Alasnag
The purpose of the ROLEX Registry (Revascularization Of LEft main with resolute onyX) was to explore outcomes using a more contemporary drug eluting stent platform which is more suitable for LM PCI.
References
Authors
Giuseppe Tarantini, Luca Nai Fovino, Ferdinando Varbella, Daniela Trabattoni, Giuseppe Caramanno, Carlo Trani, Nicoletta De Cesare, Giovanni Esposito, Matteo Montorfano, Carmine Musto, Andrea Picchi, Imad Sheiban, Valeria Gasparetto, Flavio L. Ribichini, Francesco Cardaioli, Salvatore Saccà, Enrico Cerrato, Massimo Napodano, Matteo Martinato, Danila Azzolina, Giuseppe Andò, Antonio Mugnolo, Marco Caruso, Roberta Rossini, Enrico Passamonti, Rui Campante Teles, Stefano Rigattieri, Dario Gregori, Corrado Tamburino, Francesco Burzotta
Reference
DOI: 10.4244/EIJ-D-22-00454
Published
August 31, 2022
Link
Read the abstractReviewer
My Comment
Why this study – the rationale/objective?
Current guidelines stipulate a low or intermediate Syntax score for those undergoing Left Main (LM) percutaneous coronary intervention (PCI).
Randomized studies that shaped these guidelines included the EXCEL, NOBLE, and SYNTAX trials1-3. Some observational data that capture real-world outcomes and baseline characteristics, such as the DELTA Registry are also available4.
The majority of these studies used stents that did not have a large expansion capability based on manufacturer instructions.
The purpose of the ROLEX Registry (Revascularization Of LEft main with resolute onyX) was to explore outcomes using a more contemporary drug-eluting stent platform (Resolute Onyx (Medtronic) zotarolimus-eluting stent), which is more suitable for LM PCI, with an expansion capability reaching 5.0 mm for a 3.5-4.0 mm stent and 6.0 mm for a 4.5-5.0 mm stent.
How was it executed – the methodology?
This was a prospective, international, multicenter registry of de novo LM disease treated with the Resolute Onyx zotarolimus-eluting coronary stent.
It was conducted in 26 centers in Europe. Patients above the age of 18 years with unprotected LM disease and a SYNTAX score < 33 amenable for PCI were included.
A significant LM lesion was defined as an angiographic stenosis > 50 %. If the stenosis was 50 - 70 %, a fractional flow reserve (FFR) was obtained, and hyperemia was induced using intracoronary adenosine administration.
A cut-off value of < 0.80 was set for significance. Intravascular ultrasound (IVUS) with a minimal lumen area < 6.0 mm2 was the cutoff for those who underwent further evaluation using intracoronary imaging.
Important exclusion criteria included prior revascularization, Syntax score > 33, chronic kidney disease, concomitant valvular heart disease, cardiogenic shock, and severely impaired left ventricular systolic function with left ventricular ejection fraction (LVEF) < 30 %.
The bifurcation strategy adopted was left to the discretion of the operator, with the following guidelines:
- Provisional technique was the default strategy with emphasis on proximal optimization technique (POT),
- For true LM bifurcations, an upfront two-stenting technique (T stenting, T and small protrusion [TAP], double kissing [DK] mini crush, or culotte) were employed with a final kissing balloon inflation (preferably with non-compliant balloons), and a final POT was advised but not mandated.
- Image-guided PCI (using IVUS or optical coherence tomography [OCT]) was not mandatory per protocol, but encouraged
- The other non-LM lesions were treated at the time of the index intervention or staged with the aim to achieve complete percutaneous revascularization. The primary endpoint was target lesion failure (TLF): a composite of cardiac death, target vessel myocardial infarction (TVMI), and ischemia-driven target lesion revascularisation (ID-TLR) at 1 year. The secondary endpoints were all-cause mortality, TVMI, ID-TLR, periprocedural MI, stroke rate, and (definite or probable) stent thrombosis (ST). Patients follow-up and results were reported at 30 days (±7 days) and 1 year (±30 days). Importantly, events were adjudicated by an independent clinical events committee.
What is the main result?
A total of 450 patients were enrolled, 83 % of whom were male.
The mean age was 71.8 ± 10.7 years, and the mean European System for Cardiac Operative Risk Evaluation (EuroSCORE) II was 2.7 ± 3.3. The majority had a well-preserved LV systolic function with a mean LVEF of > 50 %. NYHA Functional Class III-IV was reported in only 15 % of those enrolled.
Acute coronary syndrome (ACS) accounted for 53 % of the indications for revascularization and 37 % had prior revascularization.
In terms of co-morbidities, Diabetes mellitus was noted in 30 %, hypertension in 79 %, prior stroke in 27 %, prior MI in 25 %, peripheral vascular disease in 17 %, and chronic lung disease in 7 %.
With respect to anatomy, 36 % had a SYNTAX score < 23 and 64 % had a score between 23 and 32. 60 % had moderate calcification and 6 % had thrombus. Ostial disease accounted for 20 % of the total with distal disease accounting for the majority 78 % (Medina 1,1,1 14 %, 0,1,1 5 % and 1,0,1 8 %). Radial access was used in 77 %. IVUS was used for optimization in 42 % and OCT in 3 % and FFR in 6 %. A provisional strategy was adopted in 79 % of the patients. DK Crush was the most frequently used 2-stent technique (8 %). POT was achieved in 87 % and FKB in 64 %.
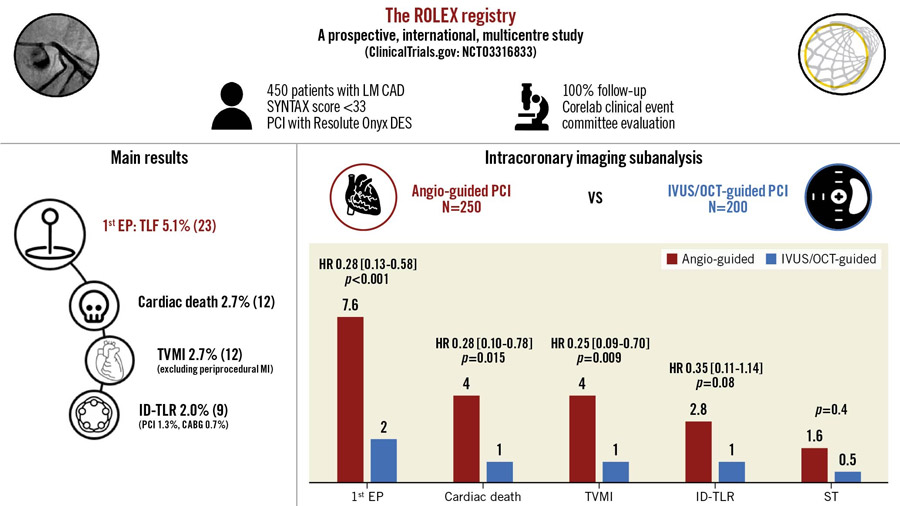
The primary endpoint occurred in 5.1 % at one year with cardiac death occurring in 2.7 % and ID-TLR in 2.0 %. Stent thrombosis was reported in 1.1 %.
As for the other secondary endpoints, BARC (Bleeding Academic Research Consortium) bleeding occurred in 4.2 %, and angina using the CCS (Canadian Cardiovascular Society Angina Score) was noted in 84 %.
The ROLEX study design and results - Source: EuroIntervention Journal
Critical reading and relevance for clinical practice
The results of this trial are aligned with previously published data, not only in terms of outcomes, but also in procedural technique.
For example, a default provisional strategy was resorted to in the majority of cases and the uptake of intracoronary imaging was also around 45 % fairly similar to DK Crush V and EBC Main trials; however, it was lower than SYNTAX and EXCEL, where use reached 70 %5-6.
Naturally, more IVUS was performed compared with OCT which is common with LM disease.
What is notable here is an analysis conducted by the investigators demonstrating much lower event rates in those who had undergone intracoronary imaging:
- Primary endpoint: intracoronary imaging-guided versus angiography-guided PCI (2.0 vs 7.6 %; 95 % CI: 0.13-0.58; p < 0.001).
- 1-year incidence of cardiac death (1.0 vs 4.0 %; 95 % CI: 0.10-0.78; p = 0.015),
- ID-TLR (1.0 vs 2.8 %; 95 % CI: 0.11-1.14; p = 0.081)
- Stent thrombosis (0.5 vs 1.6 %, p = 0.4)
The investigators did adjust for cluster in centers that perform high volume of imaging. We don’t know if those are also centers with high-volume operators treating LM bifurcation disease overall.
Interestingly, those with distal disease had similar outcomes, especially cardiac death, compared to those with isolated ostial disease.
Although the primary endpoint, ST, and ID-TLR were numerically higher in more complex disease. These are aligned with previously published data and whether the new generation stent platform with an expanding property accounted for the lower overall event rate remains speculative. We should factor in operator experience and baseline anatomy.
Moreover, the rate of ST was slightly higher in this cohort. Whether this is attributable to the lower uptake of imaging compared with EXCEL and SYNTAX cannot be ascertained.
The antiplatelet regimen was aligned with standard of care and cannot explain the higher rate of ST. These results, of course, cannot be extended to those with severely impaired LV function or CKD nor can they inform practice for those with concomitant valve disease.
An important limitation of the ROLEX registry is its inability to capture geographic differences both in terms of practice and LM size whereby the study was conducted in 26 European centers.
Finally, whether the results of ROLEX Registry will persist on longer-term follow-up is important, particularly with endpoints that did not reach statistical significance at one year.
Since a Heart Team decision was a prerequisite, ROLEX underscores the value of shared decision-making in those with LM disease particularly those who are not surgical turndowns.
References:
- Stone GW, Sabik JF, Serruys PW, Simonton CA, Généreux P, Puskas J, Kandzari DE, Morice MC, Lembo N, Brown WM 3rd, Taggart DP, Banning A, Merkely B, Horkay F, Boonstra PW, van Boven AJ, Ungi I, Bogats G, Mansour S, Noiseux N, Sabate M, Pomar J, Hickey M, Gershlick A, Buszman P, Bochenek A, Schampaert E, Page P, Dressler O, Kosmidou I, Mehran R, Pocock SJ, Kappetein AP; EXCEL Trial Investigators. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. N Engl J Med 2016;375:2223-35
- Mäkikallio T, Holm NR, Lindsay M, Spence MS, Erglis A, Menown IB, Trovik T, Eskola M, Romppanen H, Kellerth T, Ravkilde J, Jensen LO, Kalinauskas G, Linder RB, Pentikainen M, Hervold A, Banning A, Zaman A, Cotton J, Eriksen E, Margus S, Sorensen HT, Nielsen PH, Niemelä M, Kervinen K, Lassen JF, Maeng M, Oldroyd K, Berg G, Walsh SJ, Hanratty CG, Kumsars I, Stradins P, Steigen TK, Fröbert O, Graham AN, Endresen PC, Corbascio M, Kajander O, Trivedi U, Hartikainen J, Anttila V, Hildick-Smith D, Thuesen L, Christiansen EH; NOBLE study investigators. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet 2016;388:2743-528
- Mohr FW, Morice MC, Kappetein AP, Feldman TE, Stahle E, Colombo A, Mack MJ, Holmes DR Jr, Morel MA, Van Dyck N, Houle VM, Dawkins KD, Serruys PW. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013;381:629-38
- Chieffo A, Tanaka A, Giustino G, Briede I, Sawaya FJ, Daemen J, Kawamoto H, Meliga E, D'Ascenzo F, Cerrato E, Stefanini GG, Capodanno D, Mangiameli A, Templin C, Erglis A, Morice MC, Mehran R, Van Mieghem NM, Nakamura S, De Benedictis M, Pavani M, Varbella F, Pisaniello M, Sharma SK, Tamburino C, Tchetche D, Colombo A; DELTA 2 Investigators. The DELTA 2 Registry: A Multicenter Registry Evaluating Percutaneous Coronary Intervention With New-Generation Drug-Eluting Stents in Patients With Obstructive Left Main Coronary Artery Disease. JACC Cardiovasc Interv 2017;10:2401-10
- Hildick-Smith D, Egred M, Banning A, Brunel P, Ferenc M, Hovasse T, Wlodarczak A, Pan M, Schmitz T, Silvestri M, Erglis A, Kretov E, Lassen JF, Chieffo A, Lefèvre T, Burzotta F, Cockburn J, Darremont O, Stankovic G, Morice MC, Louvard Y. The European bifurcation club Left Main Coronary Stent study: a randomized comparison of stepwise provisional vs. systematic dual stenting strategies (EBC MAIN). Eur Heart J. 2021 Oct 1;42(37):3829-3839. doi: 10.1093/eurheartj/ehab283
- Chen X, Li X, Zhang JJ, Han Y, Kan J, Chen L, Qiu C, Santoso T, Paiboon C, Kwan TW, Sheiban I, Leon MB, Stone GW, Chen SL; DKCRUSH-V Investigators. 3-Year Outcomes of the DKCRUSH-V Trial Comparing DK Crush With Provisional Stenting for Left Main Bifurcation Lesions. JACC Cardiovasc Interv. 2019 Oct 14;12(19):1927-1937. doi: 10.1016/j.jcin.2019.04.056. Epub 2019 Sep 11.







No comments yet!