Long-term clinical impact of contrast-associated acute kidney injury following PCI: an ADAPT-DES sub-study
Selected in JACC: Cardiovascular Interventions by M. Alasnag , P. R. Kothapalli
The recently published ADAPT-DES sub-study addressed the question of early and long-term impact of contrast-associated acute kidney injury (CA-AKI) on net adverse clinical events. In addition, the authors defined risk factors for the development of CA-AKI in all-comers using contemporary PCI techniques.
References
Authors
Reza Mohebi, Keyvan Karimi Galougahi, Javier Jas Garcia, Jennifer Horst, Ori Ben-Yehuda, Jai Radhakrishnan, Glenn M. Chertow, Allen Jeremias, David J. Cohen, Akiko Maehara, Gary S. Mintz, Shmuel Chen, Björn Redfors, Martin B. Leon, Thomas D. Stuckey, Michael J. Rinaldi, Giora Weisz, Bernhard Witzenbichler, Ajay J. Kirtane, Roxana Mehran, George D. Dangas, Gregg W. Stone, and Ziad A. Ali
Reference
J Am Coll Cardiol Intv. 2022 Apr, 15 (7) 753–766
Published
15 April 2022
Link
Read the abstract
Reviewers
Our Comment
Why this study – the rationale/objective?
Contrast-associated acute kidney injury (CA-AKI) is well-established in the literature as a consequence of exposure during percutaneous coronary intervention (PCI).
Proposed mechanisms include direct toxic effects of contrast medium resulting in endothelial dysfunction, altered flow dynamics, or renal hypoperfusion in the setting of hemodynamic instability.
Traditional risk factors for CA-AKI include hypotension, intra-aortic balloon pump (IABP), congestive heart failure, diabetes, advanced age, anemia, contrast load, and pre-existing chronic kidney disease (CKD).
Risk calculators, such as the Mehran risk score for the prediction of contrast-induced nephropathy, have been proposed to be used in practice to predict and prevent CA-AKI1.
Furthermore, definitions of AKI vary broadly leading to misclassification of CA-AKI which also occurs as a result of cross-sectional data collection.
The incidence of post-PCI CA-AKI is roughly 7 %. Previous studies have suggested that CA-AKI is associated with significant morbidity, mortality, and hospital cost.
In a substudy of HORIZONS-AMI, a multicenter prospective randomized trial involving 3,062 patients presenting with STEMI, CI-AKI occurred in 16.1 % of patients and carried a higher risk of 30-day and 3-year net adverse clinical events and mortality2.
While several novel approaches have been trialed, the only successful strategies to reduce the risk of CA-AKI have been goal-directed intravenous hydration and minimization of the iodinated contrast load3. Despite such evidence, a transient increase in creatinine post-PCI is often dismissed by clinicians as a mild laboratory abnormality, when, in fact, the implications may be far-reaching. A critical review of our clinical practice patterns is therefore warranted.
How was it executed? - the methodology
The recently published ADAPT-DES substudy addressed the question of early and long-term impact of CA-AKI on net adverse clinical events including all-cause mortality, myocardial infarction, stent thrombosis, and major bleeding. In addition, the authors defined risk factors for the development of CA-AKI in all-comers using contemporary PCI techniques4.
This was a prospective, multicenter observational study including 11 sites in the U.S. and Germany. Baseline and post-procedure serum creatinine (within 3 days of index procedure) were available for 7,287 (85 %) of patients from the original study. CA-AKI was defined as an absolute increase of >/= 0.5 mg/dL or >/= 25 % relative increase in serum creatinine; chronic kidney disease (CKD) was defined as glomerular filtration rate < 60 ml/min/1.73m2.
What is the main result?
Of the study population, 6.5 % of post-PCI patients developed CA-AKI, with 90 % of these patients having no prior history of CKD. Clinical risk factors found to be associated with CA-AKI included female sex, peripheral arterial disease, hypertension, diabetes, congestive heart failure, anemia, and pre-existing CKD. Procedural risk factors for CA-AKI included STEMIpresentation, hypotension, IABP use, radial access, and increasing number of stents.
The authors were not able to report on the volume of contrast used, but acknowledged the number of stents as a surrogate marker for increased contrast utilization. While these findings serve to reinforce several traditional risk factors for CA-AKI, they raise an important question about the role of access site selection in contributing to this risk despite prior robust randomized data suggesting otherwise, namely the MATRIX study5. Valgimigli et al. noted no difference at one year in major adverse cardiovascular events between those assigned to radial access compared with those assigned to femoral access (14.2 % vs 15.7 %; rate ratio 0.89, 95 % CI 0.80-1.00; p = 0·0526). However, net adverse clinical events were fewer with radial than with femoral access (15.2 % vs 17.2 %; 0.87, 0.78-0.97; p = 0.0128) including CA-AKI.
Long-term clinical impact of contrast-associated acute kidney injury following PCI: an ADAPT-DES sub-study
Source : JACC - Cardiovascular Interventions
Critical reading and the relevance for clinical practice?
Reconciling this finding in the ADAPT-DES with that in the MATRIX trial is difficult given the confounders that are inherent to a subgroup analysis and changing practice patterns whereby high-risk patients are currently differentially assigned to radial access.
With respect to diabetes, over one-third of those enrolled in the ADAPT-DES were diabetics which renders them at a particularly higher risk of CA-AKI. It is difficult to extrapolate from the study whether DM per se was a driver of overall events or the mechanism of injury. Renal hypoxia and toxicity have been postulated as the underlying mechanisms. This analysis emphasizes that the overall risk in a diabetic population warrants caution and preventive measures.
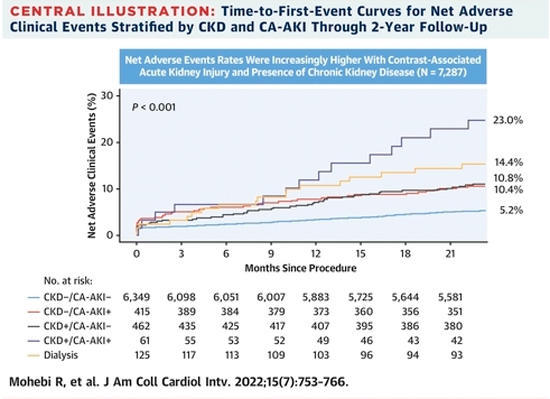
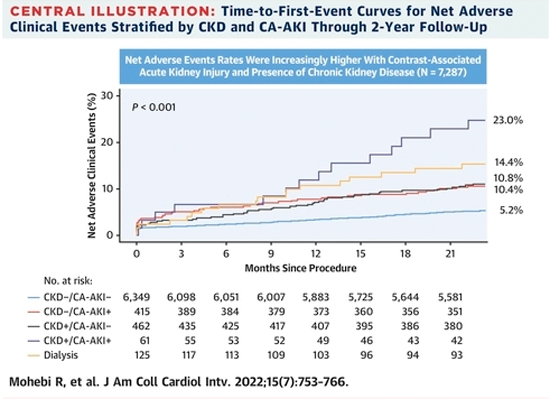
Patients with CA-AKI had increased incidence of NACE, as well as each individual component of all-cause mortality, cardiac death, myocardial infarction, stent thrombosis, and major bleeding. Pre-existing CKD placed patients in a profoundly higher risk category, with NACE occurring in 23 % of patients with CKD and CA-AKI vs. 10.4 % in non-CKD patients with CA-AKI. All-cause death in CKD patients with CA-AKI was 16.4 % while only 10.4 % in the hemodialysis CA-AKI group. An important limitation of the study is the inability to capture trends in creatinine over time, thus introducing the possibility of misclassification with early isolated measurement of creatinine.
Postprocedural acute kidney injury is notoriously associated with worse outcomes including long-term morbidity, all-cause mortality, and quality of life across the full spectrum of interventions in cardiovascular care.
This study highlights an important clinical dilemma: how can we fix the heart without ditching the kidneys? We have much to learn regarding the specific mechanisms by which injury occurs.
Further insights into these mechanisms may afford us with opportunities to prevent AKI and avert long-term complications thereof.
While novel therapeutics are being studied in this area, we as clinicians are called to recognize the substantial role we play in preventing CA-AKI in post-PCI patients by being mindful of factors that may mitigate risk in our practice.
References
- Mehran R, Owen R, Chiarito M, Baber U, Sartori S, Cao D, Nicolas J, Pivato CA, Nardin M, Krishnan P, Kini A, Sharma S, Pocock S, Dangas G. A contemporary simple risk score for prediction of contrast-associated acute kidney injury after percutaneous coronary intervention: derivation and validation from an observational registry. Lancet. 2021 Nov 27;398(10315):1974-1983. doi: 10.1016/S0140-6736(21)02326-6. Epub 2021 Nov 15. PMID: 34793743.
- Narula A, Mehran R, Weisz G, Dangas GD, Yu J, Généreux P, Nikolsky E, Brener SJ, Witzenbichler B, Guagliumi G, Clark AE, Fahy M, Xu K, Brodie BR, Stone GW. Contrast-induced acute kidney injury after primary percutaneous coronary intervention: results from the HORIZONS-AMI substudy. Eur Heart J. 2014 Jun 14;35(23):1533-40. doi: 10.1093/eurheartj/ehu063. Epub 2014 Mar 6. PMID: 24603308.
- Chau CH, Williams DO. Prevention of Contrast-Induced Renal Failure for the Interventional Cardiologist. Circ Cardiovasc Interv. 2016 Jun;9(6):e004122. doi: 10.1161/CIRCINTERVENTIONS.116.004122. PMID: 27313283.
- Mohebi R, Karimi Galougahi K, Garcia JJ, Horst J, Ben-Yehuda O, Radhakrishnan J, Chertow GM, Jeremias A, Cohen DJ, Cohen DJ, Maehara A, Mintz GS, Chen S, Redfors B, Leon MB, Stuckey TD, Rinaldi MJ, Weisz G, Witzenbichler B, Kirtane AJ, Mehran R, Dangas GD, Stone GW, Ali ZA. Long-Term Clinical Impact of Contrast-Associated Acute Kidney Injury Following PCI: An ADAPT-DES Substudy. JACC Cardiovasc Interv. 2022 Apr 11;15(7):753-766. doi: 10.1016/j.jcin.2021.11.026. Epub 2022 Mar 16. PMID: 35305904.
- Valgimigli M, Frigoli E, Leonardi S, Vranckx P, Rothenbühler M, Tebaldi M, Varbella F, Calabrò P, Garducci S, Rubartelli P, Briguori C, Andó G, Ferrario M, Limbruno U, Garbo R, Sganzerla P, Russo F, Nazzaro M, Lupi A, Cortese B, Ausiello A, Ierna S, Esposito G, Ferrante G, Santarelli A, Sardella G, de Cesare N, Tosi P, van 't Hof A, Omerovic E, Brugaletta S, Windecker S, Heg D, Jüni P; MATRIX Investigators. Radial versus femoral access and bivalirudin versus unfractionated heparin in invasively managed patients with acute coronary syndrome (MATRIX): final 1-year results of a multicentre, randomised controlled trial. Lancet. 2018 Sep 8;392(10150):835-848. doi: 10.1016/S0140-6736(18)31714-8. Epub 2018 Aug 25. PMID: 30153988.







No comments yet!