18 Oct 2022
Optical coherence tomography fractional flow reserve and cardiovascular outcomes in patients with acute coronary syndrome
Selected in JACC: Cardiovascular Interventions by N. Ryan
Optical Coherence Tomography fractional flow reserve (OCT-FFR) has been shown to correlate well with wire-based FFR; however, clinical outcomes based on OCT-FFR are not well defined. In this retrospective study, the authors sought to investigate the relationship between OCT-FFR and long-term clinical outcomes in ACS.
References
Authors
Shunsuke Kakizaki, Hiromasa Otake, Fumiyasu Seike, Hiroyuki Kawamori, Takayoshi Toba, Shinsuke Nakano, Kosuke Tanimura, Yu Takahashi, Yusuke Fukuyama, Daichi Fujimoto, Koichi Nakamura, Hiroyuki Fujii, Amane Kozuki, Junya Shite, Masamichi Iwasaki, Tomofumi Takaya, Osamu Yamaguchi, and Ken-ichi Hirata
Reference
J Am Coll Cardiol Intv. 2022 Oct, 15 (20) 2035–2048
Published
October 2022
Link
Read the abstract
Reviewer
Latest contributions
Balloon lithotripsy added to conventional preparation before stent implantation in severely calcified coronary lesions Impact of pullback pressure gradient on clinical outcomes after percutaneous coronary interventions Validation of intravascular ultrasound-defined optimal stent expansion criteria for favorable 1-year clinical outcomesMy Comment
Why this study – the rationale/objective?
The presence of residual ischaemia post PCI has been associated with poorer prognosis; however, angiography alone is limited in its ability to detect physiologically significant lesions1,2.
OCT-FFR allows assessment of the physiological significance of lesions using fluid dynamics3.
The authors aimed to determine the association between post-PCI OCT-FFR in patients with ACS and clinical outcomes in this study.
How was it executed? - the methodology
This study utilized the Kobe University ACS-OCT registry, which included patients with ACS undergoing OCT-guided PCI in four centers.
Consecutive patients enrolled from January 2010-December 2018 were retrospectively included in this analysis. Post-PCI OCT was obtained in all patients and the target vessel was divided into
- stented segment;
- adjacent reference segments (≤ 5 mm long);
- and non-culprit lesion (NCL), an untreated coronary segment with > 30 % diameter stenosis on angiography, and at least 5 mm away from the stent.
Stent and lumen areas were measured for every frame (0.1 or 0.2 mm intervals). In-stent irregular protrusion, thrombus, calcified nodule, malapposition, stent edge dissection, lipid-rich plaque (LRP), thin-cap fibroatheroma (TCFA), and macrophages were assessed for.
OCT-based suboptimal stent deployment was assessed based on the presence of at least one of the following OCT findings:
- stent underexpansion;
- in-stent plaque or thrombus protrusion ≥ 500 μm;
- malapposition > 200 μm;
- and stent edge dissection ≥ 200 μm.
OCT-FFR was calculated for the target vessel (vessel-level OCT-FFR), stented segment (stent-level OCT-FFR), and NCL (NCL-level OCT-FFR).
The primary outcome was target vessel failure (TVF), a composite of cardiac death, target-vessel-related MI, and ischaemia driven target vessel revascularization.
What is the main result?
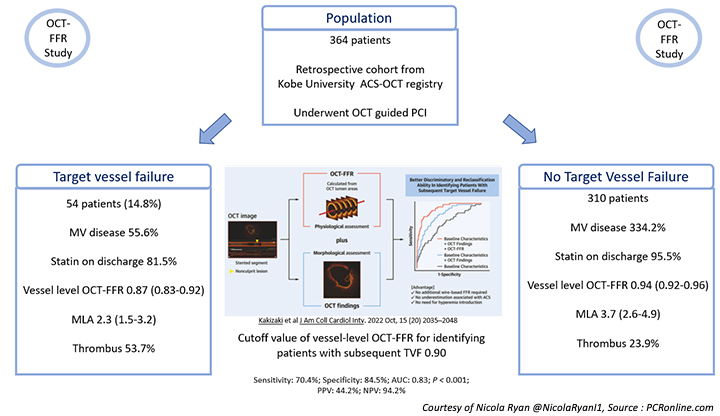
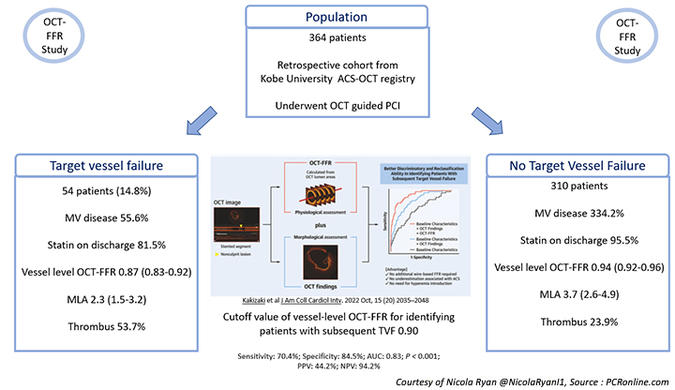
Overall, 364 patients were included in the study, 56.3 % presenting with STEMI, the majority were male in their 60’s with a high prevalence of traditional CVRFs. Over half presented with a lesion in the LAD and a third had multivessel disease.
- At a median follow-up of 36 months (IQR 26-48) TVF occurred in 14.8 % (54) of patients, 16 cardiac deaths, 33 TV MI, 39 TVR.
- Vessel level OCT-FFR was higher in the non-TVF group compared to the TVF group [0.94 (0.92-0.96) vs. 0.87 (0.83-0.92), p < 0.001).
- In the TVF group, there was more multivessel disease compared to the non-TVF group (55.6 % vs 34.2 %, p = 0.003) with statin prescription at discharge significantly lower in the TVF group (81.5 % vs. 95.5 %, p = 0.001).
- The MLA was smaller in the TVF group [2.3 (1.5-3.2) vs 3.7 (2.6-4.9), p < 0.001], with thrombus (53.7 % vs 23.9 %, p < 0.001) and irregular protrusion (70.4 % vs 52.3 % p = 0.014) more common.
- At multivariate analysis, low ejection fraction, no statin on discharge, low vessel level OCT-FFR, in-stent thrombus, lipid-rich plaque in the proximal reference segment, small average reference lumen area, and TCFA in the NCL were independently associated with TVF.
- The cutoff value of the vessel-level OCT-FFR for identifying patients with subsequent TVF was 0.90 (sensitivity: 70.4 %; specificity: 84.5 %; area under the curve: 0.83; P < 0.001; positive predictive value: 44.2 %; negative predictive value: 94.2 %).
Critical reading and the relevance for clinical practice
The results of this study show that, despite achieving angiographically acceptable PCI results, there was significant variation in post-PCI OCT-FFR values. Post-PCI OCT-FFR at a vessel level, stent level, and non-culprit lesion level, was significantly lower in patients who experienced TVF compared to those who did not. Low vessel level OCT-FFR was independently associated with TVF in patients undergoing PCI for ACS.
This study is unique in providing both a comprehensive assessment of plaque morphology, stent implantation, and physiology using an OCT imaging catheter as well as long-term clinical follow-up. The benefit of pressure wire-based FFR in the context of ACS is less well-defined than in stable coronary disease. In some cases, haemodynamic instability may lead to a reluctance to induce hyperaemia on behalf of the operator.
More importantly, micro-emboli and myocardial necrosis at the time of an acute event can lead to blunting of the hyperaemic response to adenosine and thus underestimation of the physiological significance of a lesion4. OCT-derived FFR has the advantage of not requiring a hyperaemic state as the OCT-FFR is calculated based on OCT cross-sections using fluid dynamics.
In this study, a cut-off of 0.90 identified patients at increased risk of TVF. The question that remains is: can further intervention at the time of the initial PCI improve the post-PCI OCT-FFR, and, thus, clinical outcomes?
Whilst not designed to assess optimization of post-PCI OCT-FFR multivariable analysis should that focal lesions were independently associated with low vessel-level OCT-FFR. These lesions may not have been angiographically apparent and potentially intervening on these lesions may improve vessel level post-PCI OCT-FFR and, thus, clinical outcomes.
OCT data has shown that lipid-rich plaques (LRP) and thin cap fibroatheroma in non-culprit lesions are predictive of future MACE5. This study also found that LRP in the proximal reference segment of the vessel and TCFA in the non-culprit lesions were predictive of future events. Whilst the optimal therapy for vulnerable plaque is yet to be defined identification of these features post-ACS identifies a higher-risk patient who may benefit from more aggressive medical therapy such as PCSK9 inhibitors. Importantly, whilst both the OCT findings and the OCT-FFR values were predictive of TVF combination of both increased the ability to identify patients with TVF.
A key note of caution when interpreting the results of this study is the retrospective nature of the study. Given that the initial OCT images were not specifically obtained for this study, there is variability in the lengths of the vessels imaged, and, therefore, some important OCT characteristics may not have been captured.
Whilst this study shows a relationship between OCT-FFR < 0.90 and TVF, this requires assessment in prospective clinical trials. Furthermore, understanding the features that lead to a suboptimal OCT-FFR and potential therapeutic options to improve post-PCI OCT-FFR needs further assessment and evaluation.
Overall, this study suggests that post-PCI OCT-FFR may be beneficial in identifying patients with ACS at increased risk of TVF post-PCI. Post PCI OCT-FFR adds additional predictive value above clinical and OCT features suggestive of TVF. Further prospective clinical trials assessing the value of OCT-FFR as well as role of further optimization of both stents and medical therapy with a sub-optimal OCT-FFR result are required to fully understand its place in daily clinical practice.
References
- Jeremias A, Davies JE, Maehara A, Matsumura M, Schneider J, Tang K, et al. Blinded Physiological Assessment of Residual Ischemia After Successful Angiographic Percutaneous Coronary Intervention: The DEFINE PCI Study. JACC Cardiovasc Interv. 2019 Oct 28;12(20):1991–2001.
- Piroth Z, Toth GG, Tonino PAL, Barbato E, Aghlmandi S, Curzen N, et al. Prognostic Value of Fractional Flow Reserve Measured Immediately After Drug-Eluting Stent Implantation. Circulation: Cardiovascular Interventions. 2017 Aug;10(8):e005233.
- Seike F, Uetani T, Nishimura K, Kawakami H, Higashi H, Aono J, et al. Intracoronary Optical Coherence Tomography-Derived Virtual Fractional Flow Reserve for the Assessment of Coronary Artery Disease. American Journal of Cardiology. 2017 Nov 15;120(10):1772–9.
- van der Hoeven NW, Janssens GN, de Waard GA, Everaars H, Broyd CJ, Beijnink CWH, et al. Temporal Changes in Coronary Hyperemic and Resting Hemodynamic Indices in Nonculprit Vessels of Patients With ST-Segment Elevation Myocardial Infarction. JAMA Cardiol. 2019 Aug 1;4(8):736–44.
- Kubo T, Ino Y, Mintz GS, Shiono Y, Shimamura K, Takahata M, et al. Optical coherence tomography detection of vulnerable plaques at high risk of developing acute coronary syndrome. European Heart Journal - Cardiovascular Imaging. 2021 Dec 1;22(12):1376–84.