Immediate versus staged complete revascularisation in patients presenting with STEMI and multivessel disease
Selected in EuroIntervention by A. N. Calik , M. Gökalp
The current subanalysis of the BioVasc trial offers valuable insights into the long-term effects of ICR versus SCR strategies in STEMI patients with MVD. The findings support current guidelines on the necessity of revascularizing non-culprit lesions.
References
Authors
Paola Scarparo, Jacob J. Elscot, Hala Kakar, Wijnand K. den Dekker, Johan Bennett, Manel Sabaté, Giovanni Esposito, Alberto Ranieri De Caterina, Bert Vandeloo, Paul Cummins, Mattie Lenzen, Joost Daemen,;Salvatore Brugaletta, Eric Boersma, Nicolas M. Van Mieghem, Roberto Diletti, for the BioVasc investigators
Reference
EuroIntervention 2024;20:e865-e875 published online e-edition July 2024 DOI: 10.4244/EIJ-D-23-00882
Published
Jul 15, 2024
Link
Read the abstractReviewers
Our Comment
This study is a pre-specified sub-analysis of the BioVasc trial (Percutaneous complete revascularisation strategies using sirolimus eluting biodegradable polymer coated stents in patients presenting with acute coronary syndrome and multivessel sisease).
The BioVasc trial is a prospective, open-label, randomised, multicenter, non-inferiority trial that compared immediate complete revascularisation (ICR) with staged complete revascularisation (SCR), enrolling 1,525 patients with acute coronary syndrome (ACS) and multivessel disease (MVD).
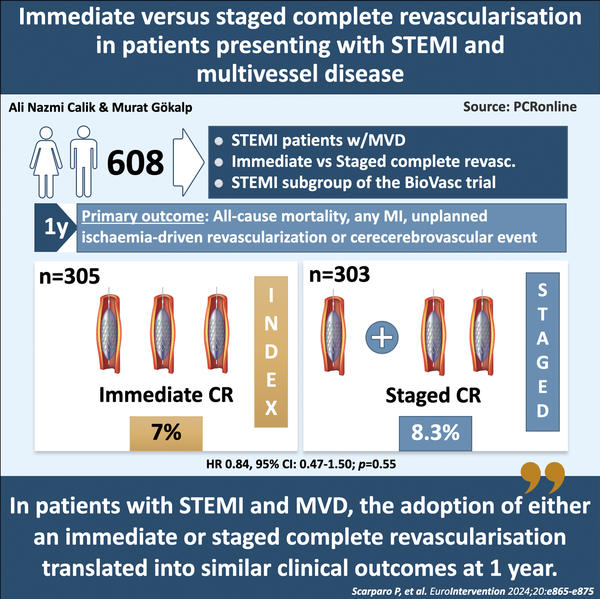
All 608 patients admitted with ST-elevation myocardial infarction (STEMI) and enrolled in the BioVasc study were included in the present analysis.
Designed by Ali Nazmi Calik & Murat Gökalp
Source: PCRonline
Why this study - the rationale/objective?
Approximately 40 % of patients with STEMI present with multivessel coronary artery disease1. It is well established that complete revascularisation, compared to percutaneous coronary intervention (PCI) of only the infarct-related artery (IRA), is superior in reducing the risks of myocardial infarction and cardiovascular mortality2. However, the optimal timing for revascularisation of non-culprit lesions in STEMI patients without cardiogenic shock remains a topic of ongoing debate.
Should these lesions be treated immediately during the index procedure (ICR), or is a staged approach (SCR) preferable?
Previous studies have shown that ICR is non-inferior to SCR regarding all-cause mortality, non-fatal myocardial infarction, stroke, and unplanned ischemia-driven revascularisation3. The BioVasc trial also supported these findings, indicating that in patients with acute coronary syndrome, ICR effectively reduces the risk of myocardial infarction and unplanned revascularisation compared to SCR4.
The aim of this prespecified analysis of the randomized BioVasc trial was to gain deeper insights into the STEMI sub-population and to evaluate the impact of ICR versus SCR on clinical outcomes in patients with STEMI and MVD.
How was it executed - the methodology
This study included patients presenting with STEMI and MVD (two or more coronary arteries with a diameter of 2.5 mm or more and ≥ 70 % stenosis based on visual estimation or positive coronary physiology testing) with a clearly identifiable culprit lesion.
Patients were randomized in a 1:1 ratio to ICR (PCI of the culprit lesion first, followed by PCI of non-culprit lesions during the index procedure) or SCR (PCI of the culprit lesion during the index procedure and PCI of all significant non-culprit lesions either during the index hospitalisation or through an elective readmission within 6 weeks after the index procedure).
The primary outcome was the composite of all-cause mortality, myocardial infarction, any unplanned ischaemia-driven revascularisation, or cerebrovascular events at 1 year after the index procedure.
Secondary endpoints comprised the primary endpoint at 30 days, individual components of the composite primary endpoint at 30 days and at 1 year, probable or definite stent thrombosis, target vessel revascularisation, and major bleeding (Bleeding Academic Research Consortium types 3 and 5) at 30 days and 1 year.
What is the main result?
Between June 2018 and October 2021, a total of 608 patients presenting with STEMI were enrolled in the BioVasc trial; 305 were randomised to ICR and 303 to SCR.
Intravascular imaging was used in 23 patients in the ICR group and in 42 patients in the SCR group (p = 0.012). Physiological assessment of the non-culprit lesion was performed in 41 patients in the ICR group and in 55 patients in the SCR group (p = 0.11). In the SCR group, 9 patients underwent physiological assessment during the index procedure and 46 patients during the staged procedure. The total procedure duration was 60 (IQR 46-80) minutes in the ICR group and 88 (IQR 64-114) minutes in the SCR group (p < 0.001). The median overall amount of contrast dye used was 200 (IQR 150-250) ml in the ICR group and 258 (IQR 200-330) ml in the SCR group (p < 0.001). The median length of the hospital stay was shorter in the ICR group compared with the SCR group (3 [IQR 2-5] days vs 4 [IQR 3-6] days; p < 0.001). In the staged group, PCI was performed as an elective procedure in 202 patients (66.7 %) and the mean time to staged procedure was 16 (3-29) days.
At 1-year follow-up, the primary composite outcome occurred in 21 (7.0 %) patients in the ICR group and in 25 (8.3 %) patients in the SCR group (HR 0.84, 95 % CI: 0.47-1.50; p = 0.55). No statistically significant differences were observed between the 2 groups in terms of all-cause mortality (ICR: 2.3 % vs SCR: 1.3 %, HR 1.77, 95 % CI: 0.52-6.04; p = 0.36), myocardial infarction (ICR: 1.7 % vs SCR: 3.3 %, HR 0.50, 95 % CI: 0.17-1.47; p = 0.21), unplanned ischaemia-driven revascularisation (ICR: 4.1 % vs SCR: 5.0 %, HR 0.80, 95 % CI: 0.38-1.71; p = 0.57), or cerebrovascular events (ICR: 1.4 % vs SCR: 1.3 %, HR 1.01, 95 % CI: 0.25-4.03; p = 0.99). Any revascularisation was significantly lower in the ICR group compared with the SCR group (4.4 % vs 8.6 %, HR 0.49, 95 % CI: 0.24-0.96; p = 0.036).
At 30-day follow-up, the primary composite outcome occurred in 9 (3.0 %) of the ICR group and in 18 (6.0 %) of the SCR group (HR 0.50, 95% CI: 0.22-1.11; p=0.09).
Critical reading and the relevance for clinical practice
The current sub-analysis of the BioVasc trial offers valuable insights into the effects of ICR versus SCR strategies in STEMI patients with multivessel disease. The findings indicate that while ICR was associated with a numerically lower incidence of the primary composite endpoint at 30 days, there was no significant difference in endpoint rates between ICR and SCR at the 1-year. Both strategies demonstrated comparable outcomes regarding all-cause mortality, myocardial infarction, and unplanned ischemia-driven revascularization over the same period.
This study contributes significantly by comparing the clinical effects of ICR with those of SCR, particularly within the context of the COMPLETE and MULTISTARS AMI trials. The COMPLETE trial indicated that the benefits of complete revascularisation are sustained with staged procedures, whether performed in-hospital or post-discharge. However, the current analysis trial did not directly compare these different staged strategies. On the other hand, the MULTISTARS AMI trial demonstrated that ICR was non-inferior to SCR for the composite primary endpoint, primarily driven by non-fatal myocardial infarction and early unplanned ischemia-driven revascularisation.
In the BioVasc sub-analysis, the adoption of an ICR approach did not exhibit a significant benefit over SCR. Despite this, the 50 % risk reduction observed in the ICR group compared to SCR at the 30-day follow-up raises the consideration of potential insufficient statistical power to detect significant differences. Nevertheless, a trend towards fewer events favoring ICR is consistent with the short-term results of the CvLPRIT, BioVasc, and MULTISTARS AMI trials.
Another significant aspect of this analysis is the investigation into the timing of staged procedures. In the MULTISTARS AMI trial, SCR was conducted between 19 and 45 days after the initial procedure, whereas in the STEMI sub-group of the BioVasc trial, a considerable portion of patients underwent revascularisation before the 19th day. This disparity implies that earlier staged procedures could have potentially averted early incidents linked to the vulnerability of non-culprit lesions. Moreover, the documented reduced overall hospital stay in the ICR group suggests possible economic advantages beyond clinical outcomes.
In summary, this study offers valuable insights into the long-term outcomes of ICR versus SCR strategies in STEMI patients with multivessel disease, presenting both clinical and economic considerations. The findings support current guidelines on the necessity of revascularising non-culprit lesions while also highlighting the potential advantages of different strategies. Therefore, this article serves as an important resource for cardiovascular practice and discussions surrounding health policy.
References
- Dziewierz A, Siudak Z, Rakowski T, Zasada W, Dubiel JS, Dudek D. Impact of multivessel coronary artery disease and noninfarct-related artery revascularization on outcome of patients with ST-elevation myocardial infarction transferred for primary percutaneous coronary intervention (from the EUROTRANSFER Registry). Am J Cardiol. 2010;106:342-7.
- Mehta SR, Wood DA, Storey RF, Mehran R, Bainey KR, Nguyen H, Meeks B, Di Pasquale G, López-Sendón J, Faxon DP, Mauri L, Rao SV, Feldman L, Steg PG, Avezum Á, Sheth T, Pinilla-Echeverri N, Moreno R, Campo G, Wrigley B, Kedev S, Sutton A, Oliver R, Rodés-Cabau J, Stanković G, Welsh R, Lavi S, Cantor WJ, Wang J, Nakamya J, Bangdiwala SI, Cairns JA; COMPLETE Trial Steering Committee and Investigators. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2019;381:1411-21.
- Stähli BE, Varbella F, Linke A, Schwarz B, Felix SB, Seiffert M, Kesterke R, Nordbeck P, Witzenbichler B, Lang IM, Kessler M, Valina C, Dibra A, Rohla M, Moccetti M, Vercellino M, Gaede L, Bott-Flügel L, Jakob P, Stehli J, Candreva A, Templin C, Schindler M, Wischnewsky M, Zanda G, Quadri G, Mangner N, Toma A, Magnani G, Clemmensen P, Lüscher TF, Münzel T, Schulze PC, Laugwitz KL, Rottbauer W, Huber K, Neumann FJ, Schneider S, Weidinger F, Achenbach S, Richardt G, Kastrati A, Ford I, Maier W, Ruschitzka F; MULTISTARS AMI Investigators. Timing of Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2023;389:1368-79.
- Diletti R, den Dekker WK, Bennett J, Schotborgh CE, van der Schaaf R, Sabaté M, Moreno R, Ameloot K, van Bommel R, Forlani D, van Reet B, Esposito G, Dirksen MT, Ruifrok WPT, Everaert BRC, Van Mieghem C, Elscot JJ, Cummins P, Lenzen M, Brugaletta S, Boersma E, Van Mieghem NM; BIOVASC Investigators. Immediate versus staged complete revascularisation in patients presenting with acute coronary syndrome and multivessel coronary disease (BIOVASC): a prospective, open-label, non- inferiority, randomised trial. Lancet. 2023;401:1172-82.






No comments yet!