Percutaneous coronary treatment with Bioadaptor implant vs drug-eluting stent : 2-year outcomes from BIOADAPTOR RCT
Selected in JACC: Cardiovascular Interventions by G. Occhipinti , S. Brugaletta
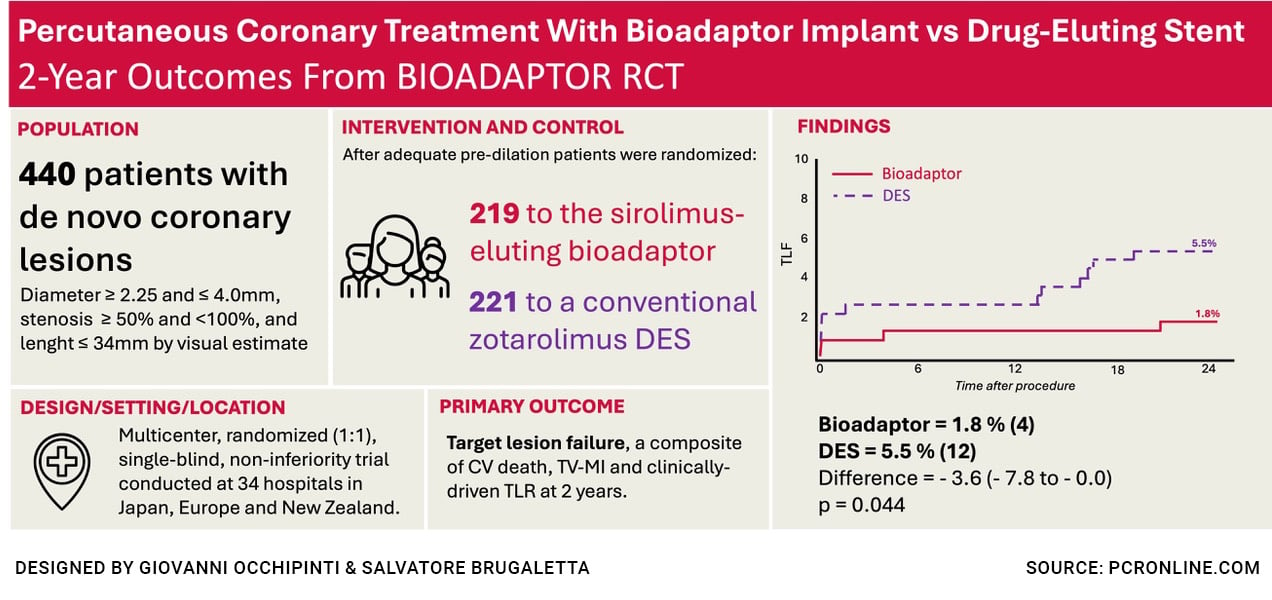
At two-year follow-up, the use of sirolimus-eluting cobalt-chromium DynamX Drug Eluting Coronary Bioadaptor System (Elixir Medical Corporation) resulted in fewer target lesion failure (TLF) compared with the Resolute Onyx contemporary drug-eluting stent.
References
Authors
Shigeru Saito, Johan Bennett, Holger M. Nef, Mark Webster, Atsuo Namiki, Akihiko Takahashi, Tsunekazu Kakuta, Seiji Yamazaki, Yoshisato Shibata, Douglas Scott, Mathias Vrolix, Madhav Menon, Helge Möllmann, Nikos Werner, Antoinette Neylon, Zlatko Mehmedbegovic, Pieter C. Smits, Marie-Claude Morice, Stefan Verheye; the BIOADAPTOR-RCT Collaborators
Reference
J Am Coll Cardiol Intv. Mar 08, 2025. Epublished DOI: 10.1016/j.jcin.2025.01.426
Published
March 08th, 2025
Link
Read the abstractReviewers
Our Comment
Designed by Giovanni Occhipinti & Salvatore Brugaletta. Source: PCRonline
Why this study – the rationale/objective?
To provide the first randomised clinical evidence comparing 2-year clinical outcomes of the DynamX sirolimus-eluting Bioadaptor system with a contemporary drug-eluting stent (DES).
How was it executed – the methodology?
The BIOADAPTOR RCT was a multicenter, randomised, single-blind (patients blinded to device allocation), controlled trial. Eligible patients had de novo coronary artery lesions (visual stenosis ≥ 50 % and < 100 %), vessel diameter between 2.25 mm and 4.0 mm, and lesion length ≤ 34 mm.
Following successful lesion preparation, patients were randomised to receive the DynamX bioadaptor or the Resolute Onyx DES. Primary endpoint was target lesion failure (TLF), a composite of cardiovascular death, target vessel-myocardial infarction (TV-MI), and clinically driven-target lesion revascularisation (CD-TLR) at 12 months.
This analysis reports 2-year outcomes.
What is the main result?
At 2 years, the Bioadaptor group exhibited fewer TLFs than the DES group [1.8 % vs 5.5 %; risk difference -3.6 % (95 % CI: -7.8 % to -0.0 %; P = 0.044)].
The primary endpoint occurred in 4 subjects (1.8 %) assigned to Bioadaptor group vs. 12 subjects (5.5 %) assigned to the control group. Results were consistent also in the intention-to-treat (ITT) population [2.3 % vs 5.4 %; risk difference of -3.2 % (95 % CI: -7.4 % to 0.6 %; P = 0.093)], and across different subgroups.
Cardiovascular death did not occur in the Bioadaptor group, but occurred in 4 patients in the control group [0.0 % vs 1.8 %; risk difference -1.8 % (95 % CI: -0.6 % to 0.0 %; P = 0.044)]. No late or very late stent thrombosis was observed in either group through 2 years, consistent in both per-protocol and ITT analyses.
Critical reading and the relevance for clinical practice
Stent-related adverse events continue to increase at a rate of 2-3 % a year after the first 12 months. The “leave nothing behind” concept of bioresorbable scaffolds and drug-coated balloons (DCBs) failed to improve outcomes compared to DES.
In this area, the Bioadaptor has the potential to avoid device-related adverse events through a technology able to both achieve flow restoration while preserving vessel pulsatility and compliance. Based on the results of the BIOADAPTOR RCT and the premises of the unique device design, it may be argued that the journey of the Bioadaptor device is promising.
However, several points should be considered. Patients with acute coronary syndrome within the previous seven days were excluded from the study, as were those with complex coronary artery lesions. The number of screening failures was not reported, raising questions about the real-world feasibility of an upfront Bioadaptor-based strategy. Finally, operator blinding was not feasible due to the visual differences between the devices: this setting could have introduced procedural bias at the index procedure related to heightened attention on achieving optimal angiographic results with a novel device with subsequent potential role in outcome.
It should be also noted that the primary endpoint was powered for 1-year outcomes, it did not show any superiority; therefore, the 24-month outcomes should be considered hypothesis-generating. Looking at the primary outcome, the difference between the two groups appears to be driven by lower cardiovascular death, and not by TV-MI and CD-TLR, areas in which the Bioadaptor’s design might have been expected to yield a benefit.
Nevertheless, the present findings should be evaluated together with those of the INFINITY-SWEDEHEART trial, which demonstrated the non-inferiority of Bioadaptor in terms of TLF at 12-month compared to DES, showing the potential to mitigate non-plateauing device-related events.
Importantly, the trial also included patients with complex coronary anatomy and acute coronary syndrome, broadening the potential applicability of this technology. Taken together, the clinical relevance of these findings remains debated, and the question is open for further investigation.
Finally, the results from the SCAAR Registry comparing real-world outcomes of Bioadaptor versus DCB will be also available, potentially offering critical insights and contributing further to this evolving field.







No comments yet!