Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel
Selected in The New England Journal of Medicine (NEJM) by L. Biasco
An active surveillance programme was initiated by the Israeli Ministry of Health to ascertain the possible causal relationship between the vaccine and myocarditis and evaluate incidence, clinical relevance, and related outcomes.
References
Authors
Dror Mevorach, Emilia Anis, Noa Cedar, Michal Bromberg, Eric J. Haas, Eyal Nadir, Sharon Olsha-Castell, Dana Arad, Tal Hasin, Nir Levi, Rabea Asleh, Offer Amir, Karen Meir, Dotan Cohen, Rita Dichtiar, Deborah Novick, Yael Hershkovitz, Ron Dagan, Iris Leitersdorf, Ronen Ben-Ami, Ian Miskin, Walid Saliba, Khitam Muhsen, Yehezkel Levi, Manfred S. Green, Lital Keinan-Boker, and Sharon Alroy-Preis
Reference
N Engl J Med. 2021 Oct 6. doi: 10.1056/NEJMoa2109730. Epub ahead of print. PMID: 34614329.
Published
October 6, 2021
Link
Read the abstractReviewer
Latest contributions
TAVI complications - Part 5 Unusual structural interventions: infective endocarditis, ventricular septal rupture & hypertrophic obstructive cardiomyopathy Tendyne transcatheter mitral valve system in patients with severe mitral annular calcification: one-year outcomes from the SUMMIT Severe MAC cohortMy Comment
Why this study? – the rationale/objective
After the initiation of a nationwide vaccination campaing with BNT162b2 mRNA (Pfizer-Biontech) vaccine against Covid-19 in Israel, and the recognition of initial signals indicating an increased incidence of myocarditis in close temporal relationship with vaccinations, an active surveillance programme was initiated by the Israeli Ministry of Health to ascertain the possible causal relationship between the vaccine and myocarditis and evaluate incidence, clinical relevance, and related outcomes.
How was it executed? - the methodology
Since February 2021, Israeli Ministry of Health initiated an active surveillance programme by requesting health care providers to report data on cases of all myocarditis cases (with or without concomitant pericarditis) diagnosed since December 2020.
A hybrid retrospective (December 2020-February 2021) / prospective (February-May 2021) cohort study was designed and cases were screened starting from administrative records (codes for myocarditis 422.0-9 x and 429.0 x of the International Classification of Diseases, 9th Revision).
After this initial screening, each identified case was adjudicated by a board-certified cardiologist and rheumatologist through medical records revision and evaluated as definitive, probable, possible, having insufficient data, or having an alternative diagnosis according to the Brighton Collaboration Myocarditis definition (Myocarditis/Pericarditis Case Definition - Brighton Collaboration).
In order to ascertain the close temporal relationship with COVID-19 vaccination incidence of myocarditis during the first 21 days after the first dose of vaccine and 30 days after the second dose was evaluated.
Study period was defined from December 2020 to May 2021.
Finally, incidence of myocarditis observed in the predefined study period was compared to the background, expected, incidence as estimated from data extracted from the national discharge database for the years 2017 through 2019 and that observed among non vaccinated residents.
What is the main result?
- Among more than 9 million residents evaluated in the surveillance period, (5,442,696 after a first dose and 5,125,635 after a second dose), 142 received a diagnosis of myocarditis in close temporal proximity with BNT162b2 mRNA vaccine administration. After medical records revision, 136 were defined as definite or probable myocarditis, 1 as possible myocarditis, while in 5 data were insufficient to define the diagnosis according to the Brighton Collaboration Criteria.
- 101 cases of myocarditis were reported in the study period among non-vaccinated patients (in 29 cases, a COVID-19 related myocarditis was ascertained).
- 129 out of 136 (95 %) with definite or probable myocarditis diagnosed in close temporal proximity with vaccination had an uneventful clinical course with short hospital stay. One case of fatal fulminant myocarditis was observed.
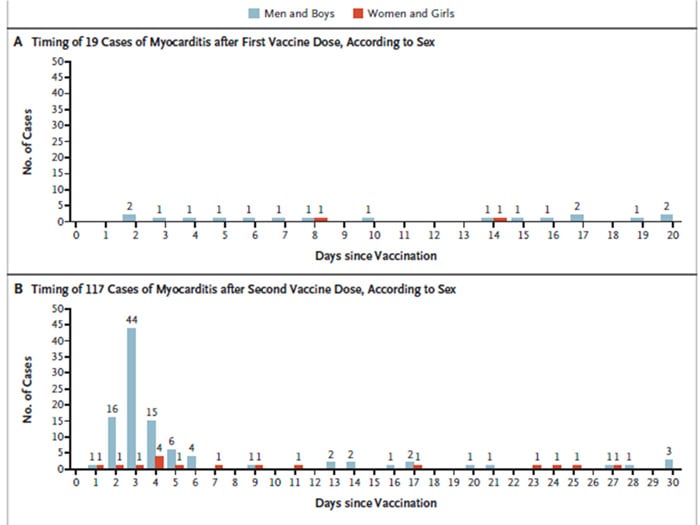
- While only 19 cases presented after the first dose, 117 were reported following administration of the second dose, with cases clustering in the 5 days following the second dose.
- Overall, no significant differences were evident between observed and expected cases of myocarditis after a first BNT162b2 mRNA dose [observed cases 25 vs expected cases 17.55, standardized incidence ratio 1.42 (0.92–2.10) ]. While, after a second dose, 126 cases were recorded as compared to 23.43 expected [standardized incidence ratio 5.34 (4.48–6.40)], this driven by a greater incidence in males among all age groups.
- When compared to non vaccinated, 117 myocarditis cases were observed in BNT162b2 mRNA recipients over 149,786,065 person/days follow up as compared to 98 cases over 296,377,727 person/days follow up [Risk Ratio 2.36 (1.10-5.32)]. Also, in this case, difference was driven by cases recorded among young males (age < 29 years).

Source NEJM: Timing and Distribution of Myocarditis after Receipt of the BNT162b2 Vaccine. Shown is the timing of the diagnosis of myocarditis among recipients of the first dose of vaccine (Panel A) and the second dose (Panel B), according to sex.
Critical reading and the relevance for clinical practice
Occurrence of myocarditis with a close temporal relationship with vaccination is a rare but known adverse event already described after smallpox, influenza, hepatitis B and other vaccinations.
While phase III trials were not powered to recognize differences among rarely encountered adverse events such as myocarditis, epidemiological surveillance programmes developed during the current worldwide vaccination campaign, reported on the potential occurrence of myocarditis after vaccination with both BNT162b2 (Pfizer–BioNTech) and mRNA 1273 (Moderna) Covid-19 vaccines. In fact, after a first alert by the US Center for Disease Control, more recently, two Israeli simultaneous publications on the NEJM focused on this topic1-4.
The present work reports on the occurrence of myocarditis in close temporal relationship with BNT162b2 Covid-19 vaccination in Israeli residents, by providing a comparative analysis with expected cases according to the incidence observed between 2017 and 2019, as well as the simultaneous incidence observed in unvaccinated subjects. This analysis adds evidence allowing to infer regarding causality between the mRNA vaccination and subsequent development of myocarditis.
From a clinical perspective, the clinical course on a short-term follow-up seems to be benign, as in most cases, patients had prompt improvement of symptoms with medical therapy and a short in-hospital stay. Nonetheless, sporadic fatal fulminant cases have been reported both from Mevorach et al. and Witberg et al3,4.
Imaging data on left ventricular functional impairment are still scarce, but overall, complete restoration of LV dysfunctions seems common.
No data are available on the long-term impact of mRNA vaccination-related myocarditis.
Pathophysiological pathways behind mRNA associated myocarditis are not fully understood but two main mechanisms are called upon. At first, a certain degree of activation of innate immunogenicity in susceptible individuals might be triggered by mRNA injection, causing the production of neutralizing anti-mRNA antibodies, leading to autoantibody generation. Alternatively, molecular mimicry between SARS-CoV2 spike protein and certain self-antigens (such as alpha myosin) might justify an autoimmune response and myocardial inflammation2.
While no clear explanation exists for the increased occurrence in younger males, evident also in non-vaccine-related myocarditis, a possible explanation might lay in the pro-inflammatory effects of Testosterone.
Interestingly, cases are clustered a few days after a second dose (most commonly on the second to sixth day) allowing clinicians to have a valuable clue to formulate a working diagnosis based on clinical history.
Even if administration of a third “booster dose” is now hypothesized for elderly frail patients and subjects at high risk of contact with SARS-CoV2 (such as health care professionals), attention should be paid in particular in the latter, usually younger group, in order to adequately monitor and report all cases of myocarditis and pericarditis post-COVID-19 vaccination boost.
Despite these supportive data regarding the association between mRNA based vaccines and myocarditis, the risk-benefit ratio remains clearly in favour of immunization. According to CDC-derived data, the potential risk of myocarditis in subjects aged 18-24 has an estimated incidence rate of 4 to 5 cases in females and 45-56 in males for every million second dose of COVID-19 mRNA vaccination.
On the other, every million complete vaccination in the same population strata prevents 14,000 COVID-19 infections, 1,127 hospital admissions, 93 ICU admission, and 13 deaths in females, and 12,000 COVID-19 infections, 530 hospital admissions, 127 ICU admission, and 3 deaths in males.
Following initial the recognition of the prognostic role of endothelial involvement and myocardial injury in COVID-19 patients, and the current evidence of immuno-mediated myocarditis following mRNA vaccination, the heart and cardiovascular system have been put at the centre of a complex, and still largely unknown, immune network that might hopefully provide insights into several pathophysiologic pathways with potential therapeutic targets.
References
- Center for Disease Control and Prevention (CDC) Advisory Committee in Immunization practice. Coronavirus Disease 2019 (COVID-19) https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html
- Bozkurt B, Kamat I, Hotez PJ. Myocarditis With COVID-19 mRNA Vaccines. Circulation. 2021 Aug 10;144(6):471-484. doi: 10.1161/CIRCULATIONAHA.121.056135. Epub 2021 Jul 20. PMID: 34281357; PMCID: PMC8340726.
- Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, Grinberg T, Auster O, Dagan N, Balicer RD, Kornowski R. Myocarditis after Covid-19 Vaccination in a Large Health Care Organization. N Engl J Med. 2021 Oct 6. doi: 10.1056/NEJMoa2110737. Epub ahead of print. PMID: 34614329.
- Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, Olsha-Castell S, Arad D, Hasin T, Levi N, Asleh R, Amir O, Meir K, Cohen D, Dichtiar R, Novick D, Hershkovitz Y, Dagan R, Leitersdorf I, Ben-Ami R, Miskin I, Saliba W, Muhsen K, Levi Y, Green MS, Keinan-Boker L, Alroy-Preis S. Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N Engl J Med. 2021 Oct 6. doi: 10.1056/NEJMoa2109730. Epub ahead of print. PMID: 34614328.




No comments yet!