TAV-in-SAV: transcatheter heart valve (THV) selection
Transcatheter aortic valve implantation within failing surgical aortic bioprostheses
After your patient has been carefully selected, the choice of which transcatheter heart valve (THV) to implant within a failed surgically implanted bioprosthetic valve should be highly tailored for each individual case. A good knowledge of the different THVs available and an awareness of the different complications involved is thus essential. This section looks at the various choices in the interventionists’ armamentarium today, focusing on the different valves, the question of sizing and the various procedures used in implanting them…
The choice of THV for implantation should be individualised for each patient (Movie 31) and (Movie 32) below. In the majority of cases, the Edwards SAPIEN (Figure 8) or CoreValve (Figure 9) are likely to be equally efficacious. Patients with bioprostheses of small (<20 mm) internal diameter may benefit from the superior haemodynamic results associated with the CoreValve31. Consideration of the risk of coronary ostial occlusion may also influence THV selection. New-generation, fully retrievable THV devices or those with aortic leaflet clipping (JenaValve; JenaValve Technology GmbH, Munich, Germany) may be preferable if the risk of coronary occlusion is deemed to be high. Furthermore, if the risk of coronary occlusion seems high, a safety wire (with/without a loaded stent) can be placed in the coronary arteries to facilitate and/or expedite percutaneous coronary intervention (Movie 25 and Figure 10).
Transfemoral implantation of a 26mm Medtronic CoreValve inside a stenotic and regurgitant St Jude Toronto stentless bioprosthesis. Note the heavy calcification of the surgical bioprosthesis.
Transapical implantation of 26mm Edwards SAPIEN XT inside a stenotic Sorin Mitroflow bioprosthesis.
Edwards SAPIEN TAV-in-SAV procedure.

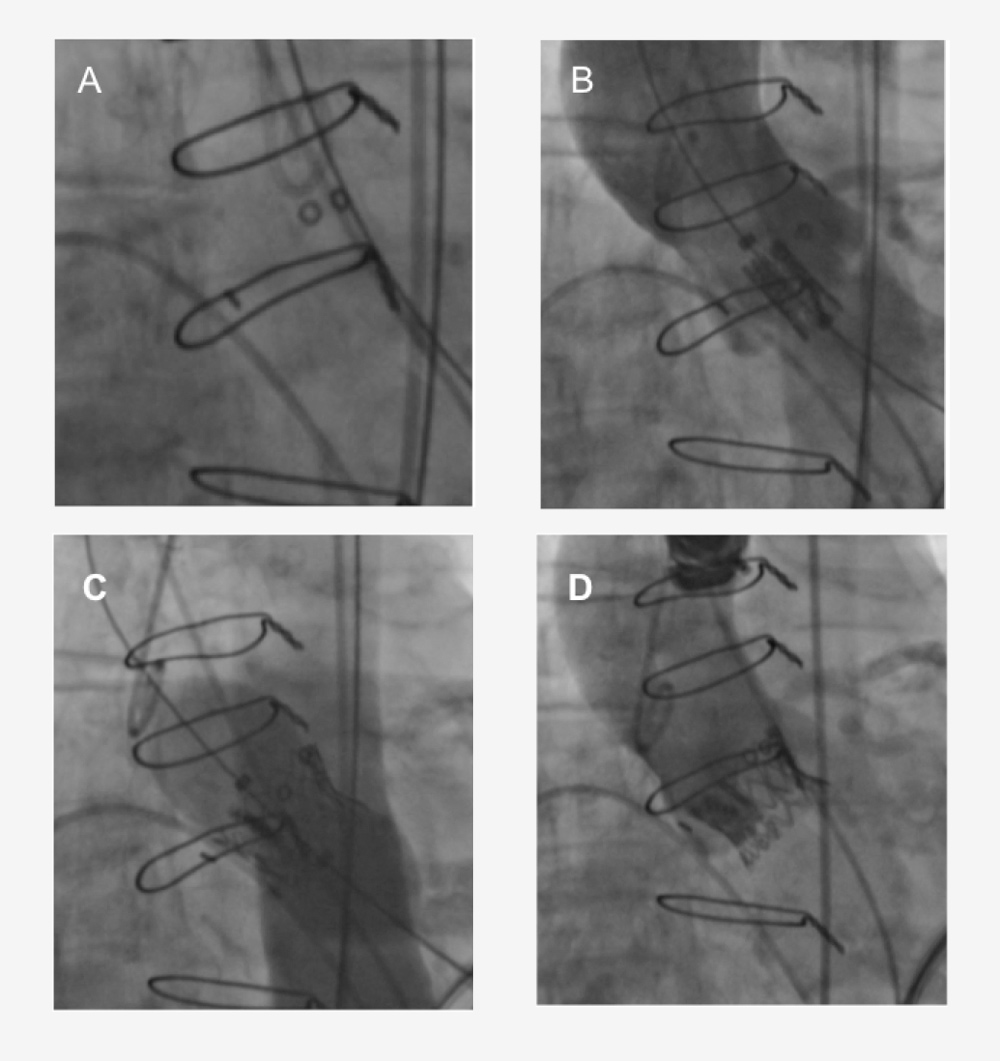
Figure 9. Transapical implantation of a 23 mm Edwards SAPIEN TAV inside a 21 mm Medtronic Mosaic bioprosthesis. (A) Fluoroscopic identification of the Medtronic Mosaic valve. (B) Positioning of the Edwards SAPIEN valve. (C) Deployment of the Edwards SAPIEN valve. (D) Final contrast aortography. With permission from Mylotte et al. Heart. 2013 99:960-7

Figure 10. Medtronic CoreValve prosthesis implantation within (A) Edwards Perimount, (B) Carpentier-Edwards Porcine supra-annular valve, and (C) Sorin Soprano bioprosthesis. With permission from D. Mylotte et al. Heart. 2013 99:960-7
Transfemoral implantation of a Medtronic CoreValve within a stenotic Sorin Mitroflow surgical bioprosthesis. No balloon pre-dilatation was performed. Note the 0.014 coronary guidewire in the left anterior descending coronary artery.
Post implantation aortography demonstrates patency of the left main coronary artery and trivial paravalvular leak.
Additional links
References - TAVI Atlas: TAVI for failing surgical aortic bioprostheses