One-year results of the Clasp TR Study: Transcatheter treatment of tricuspid regurgitation
Reported from ACC 2022
Alessandro Sticchi and Luigi Biasco provide their take on the 1-year results of the CLASP TR study which were presented by Dr Adan Greenbaum, co-director of the Emory Structural Heart and Valve Center (Atlanta, GA) during the 2022 American College of Cardiology annual Congress held in Washington D.C.
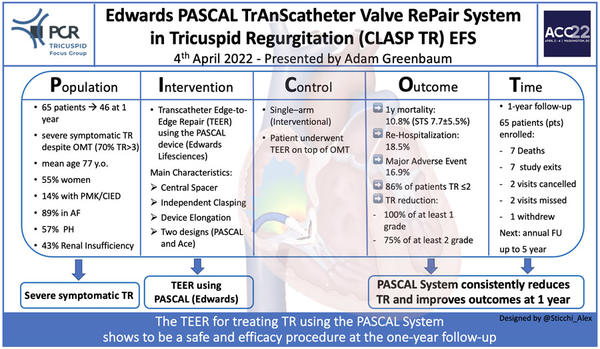
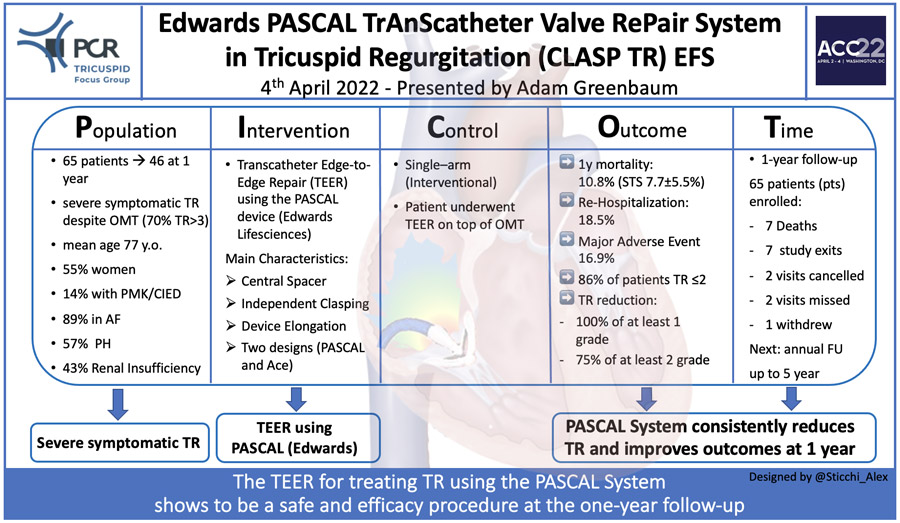
This is an early feasibility study to assess safety and efficacy of the Edwards PASCAL Transcatheter Edge-to-Edge Repair (TEER) System for the treatment of patients with severe symptomatic tricuspid regurgitation (TR). Presented data extend the short-term results previously published by Kodali S. et al in J Am Coll Cardiol. 2021;77(4):345-356.
PICOT analysis by Alex Sticchi
Why this study? – the rationale/objective
TR is a prevalent disease affecting approximately 4% of subjects aged above 75 years. While considered an un-relevant valvular disease with modest prognostic impact for a long time, recent works have clearly demonstrated its detrimental effect and a ten-year mortality exceeding 75%.
The unsatisfactory clinical results observed with conservative medical therapy, the high surgical procedural risk associated with isolated TR (estimated to reach 10% in retrospective series) leading to a low number of surgical procedures performed annually, even among high surgical volume centres, lead to the search for transcatheter options.
This study aimed to assess the feasibility and safety of the PASCAL transcatheter valve repair system in TR.
How was it executed? - the methodology
The Edwards CLASP TR trial is an early feasibility, prospective, single-arm, multicentre US study including 65 patients (with a mean age of 77±9 years (STS score for MV repair 7.7%±5.5%; EuroScore II 5.0±4.7) with baseline severe symptomatic (dyspnoea class NYHA III/IV [71%]) TR (70% massive and torrential [39%]) underwent Transcatheter with the investigational device. No data were provided on the number of patients screened before the enrollment.
The main inclusion criteria were:
- Severe functional or degenerative TR in patients symptomatic despite medical therapy.
- Appropriate anatomy for percutaneous transcatheter tricuspid valve repair as assessed by the local site Heart Team.
Major exclusion criteria were defined as:
- Presence of anatomic features precluding proper implant such as
- septolateral coaptation gap >10 mm,
- leaflet length <8 mm,
- calcifications in the annulus, leaflets or grasping areas,
- pacemaker lead induced TR preventing proper device placement,
- previous TV repair that would interfere with implant placement,
- TR associated with carcinoid disease or of rheumatic, endocarditis, traumatic, iatrogenic aetiologies.
- Left ventricular ejection fraction (LVEF) <30%,
- severe right ventricular dysfunction (core lab assessed),
- pulmonary systolic pressure >60mmHg (core lab assessed),
- severe concomitant valve disease,
- End stage renal disease with need of renal replacement therapy or estimated glomerular filtration rate <30 ml/min/1.73 m2.
- Previous tricuspid valve repair or replacement
- Co-morbid condition(s) limiting the patient's ability to participate in the study
The primary endpoint is a composite of Major Adverse Events (cardiovascular mortality, stroke, myocardial infarction, intervention, either percutaneous or surgical, major access site and vascular complications, renal complications requiring unplanned dialysis or renal replacement therapy, and severe MVARC defined bleeding).
Additional 1-year outcome measures were also specified as:
- Improvement in NYHA Functional Class.
- Change in distance at six minutes walking test.
- Reduction in TR grade from baseline.
- Improvement of health status as measured by Kansas City Cardiomyopathy and SF-36 questionnaires.
This study has been funded by the sponsor (Edwards Lifesciences), with active participation of on-site selection, data collection and monitoring, and statistical analysis. The study included a central screening committee and an echocardiographic core laboratory. All events were adjudicated by a clinical events committee.
Major findings
Forty-six patients completed 1-year follow-up. Reasons for study exit were death (n=7), study exit (n=7), missed /not due visit (n=4), consent withdrew (n=1).
Successful implant rate (defined as implant deployed and system retrieved at case completion) was obtained in 91% of patients. However, the procedure was aborted in six patients due to complex tricuspid anatomy impeding proper device implant with investigational devices successfully retrieved. Rarely more than two devices were needed (46%: 1 device; 41%: two devices).
Procedural success rate (defined as implantation with at least > 1-grade reduction in TR at its completion) has been obtained in the 88% of patients. In addition, procedural success without major adverse events at 30 days occurred in 77% of the population.
The mean reported procedural time was 147±89 minutes, while the mean hospital stay was 2.6 days.
At 1-year follow-up, five cardiovascular deaths were observed (7.7%; all-cause mortality n=7, 10.8%), while major adverse events were observed in 11 patients (16.9%) with severe bleeding resulting as the more frequent adverse event at follow-up.
Surgical device explantation was needed in one patient, and a successful surgical tricuspid repair was achieved by implanting a surgical ring. Partial device detachment was recorded in 3 patients (core-lab confirmed). Recurrent HF hospitalizations were recorded in 12 patients (18.5%).
At 1-year follow-up, core-lab assessed TR severity resulted ≤ moderate in 86% (p<0.001 at paired analysis). Reduction of volume overload resulted in a positive right atrial and ventricular remodelling with a significant reduction in tricuspid annulus diameter, RV end-diastolic diameter, RA volume and inferior vena cava diameter. Moreover, the study showed a clinical improvement in NYHA class ≤ 2 in 92% of patients and a significant increase of 94 m at 6MWD. As measured by Kansas City Cardiomyopathy (all p<0.05 at 1-year paired analyses), significant improvement in health status was also reported.
Critical reading and the relevance for clinical practice
This important study is the very first step in the validation of the PASCAL TEER for treating TR. As we previously described, it confirms the safety and the efficacy of the Edwards’ device at the one-year follow-up.
The small number of the CLASP study and the small data available in the long-term need caution about a definite statement, but several certainties are here to stay.
Due to the rapid and intense evolution in this field, the experience of several Heart-Valve High-volume centres, both in the US and Europe, is currently beyond the results of this study. The TEER is the most widespread and applied device for treating TR and its technical evolution together with the growing experience brings us a new favourable horizon in Tricuspid valve repair on the improvement in echographic, functional outcomes and quality of life.
The last ESC Valvular Guidelines included a recommendation (IIb C: may be considered) for treating symptomatic secondary severe TR using TEER in patients not suitable for surgery. This study reinforces this recommendation and represents another important step in the confirmation and understanding of this therapy.
In high-volume Tricuspid dedicated centers and under experienced hands, the initial long procedural time comes to a sensible reduction. Moreover, the improvements in the device technology and in the imaging approach, especially with the ICE-4D, will determine a considerable step forward in this hot topic.
Further data will come from the CLASP II TR (NCT04097145) randomized trial which is currently recruiting (estimate enrollment: 825 patients) and will compare the PASCAL system versus optical medical therapy.
The future of Tricuspid treatment is shining more than ever.
Authors





No comments yet!