The JenaValve Trilogy™ heart valve system in high surgical risk patients with symptomatic, severe aortic regurgitation: The ALIGN AR Trial
Reported from TCT 2023
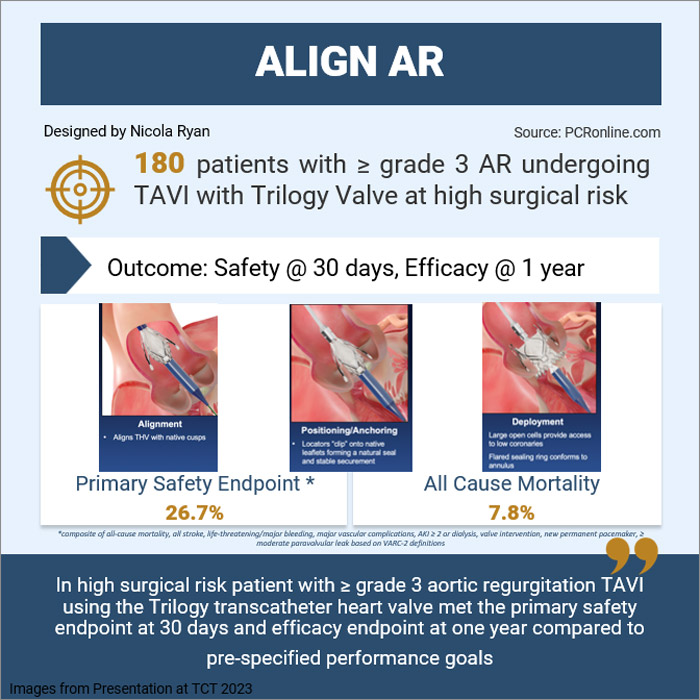
Nicola Ryan provides her take on the ALIGN AR trial which was presented by Vinod H. Thourani at TCT Congress 2023. The Align AR trial is a prospective single-arm investigation device exemption study assessing the safety and efficacy of the Trilogy transcatheter heart valve system in patients with severe symptomatic aortic regurgitation at high surgical risk.
Note the assumptions in this article are based on the presentation alone as the trial has not yet been published.
Why this study – the rationale/objective?
Untreated severe aortic regurgitation has a high morbidity and mortality however in a not insignificant number of patients the surgical risk is deemed to outweigh the benefits. TAVI with off-label devices in patients with pure aortic regurgitation is challenging due to the absence of calcification leading to an increased risk of valvular embolization, migration or paravalvular leak. The Trilogy valve was designed for patients with pure AR with alignment to the native cusps, locators which clip onto the native leaflets to form a seal and secure the device in a stable position and a flared sealing ring which conforms to the native annulus following deployment. The Align AR trial is a single-arm prospective investigation device exemption study assessing the safety and efficacy of the JenaValve Trilogy transcatheter heart valve system in patients with severe symptomatic aortic regurgitation at high risk for SAVR.
How was it executed - the methodology?
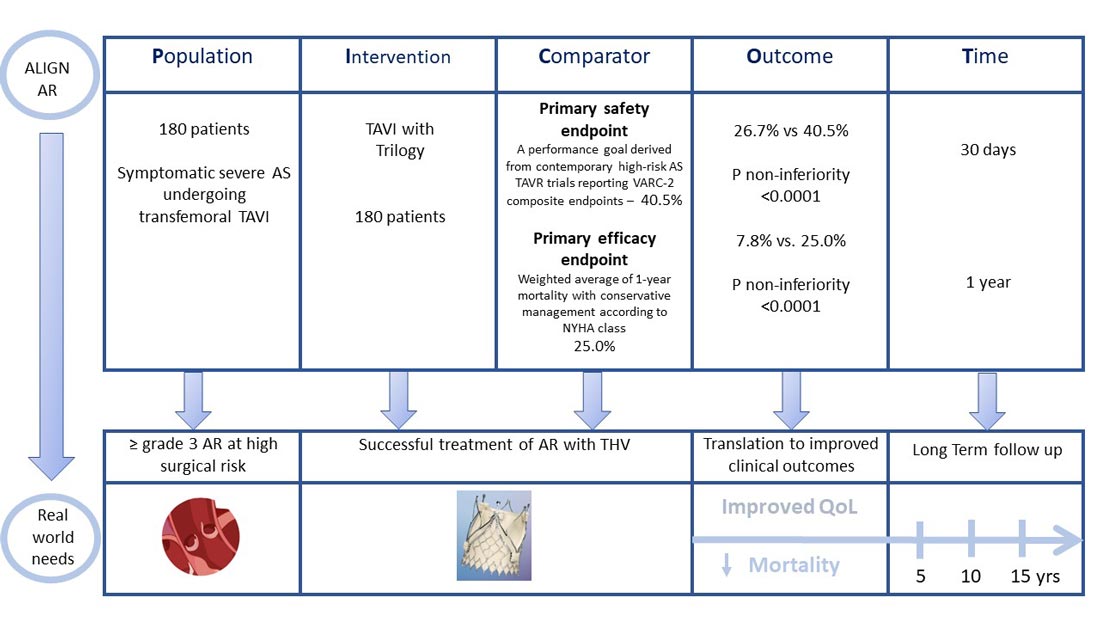
The Align AR trial included symptomatic (NHYA II or greater) patients with grade ≥3+ AR at high surgical risk as assessed by the local heart team and the study case review board. Key exclusion criteria included a uni or bicuspid valve, an aortic root >5.0cm, previous prosthetic AV and CAD requiring revascularisation.
- The primary safety endpoint was a composite of all-cause mortality, all stroke, life-threatening/major bleeding, major vascular complications, AKI ≥ 2 or dialysis, valve intervention, new permanent pacemaker, ≥ moderate paravalvular leak based on VARC-2 definitions at 30 days
- The primary efficacy endpoint was all-cause mortality at 12 months.
- Key secondary endpoints included: cardiovascular mortality, disabling stroke, haemodynamic valve performance, LV remodelling, NHYA functional class and quality of life.
The comparator for the primary safety endpoint was a performance goal which was derived from contemporary high-risk AS TAVR trials reporting VARC-2 composite endpoints – This was set at 40.5%.
The comparator for the primary efficacy endpoint was derived as a weighted average of 1-year mortality with conservative management according to NYHA class – This was set at 25.0%.
What is the main result?
Overall, 180 patients were enrolled in the Align AR trial with 177 undergoing successful implantation of the Trilogy valve (one converted to SAVR, 2 implantation of commercial TAVI valves). Almost 50% of the patients were female with a mean age of 75 years, two-thirds of patients were NYHA III or IV with a mean STS score of 4.1. By imaging criteria, 64.4% had severe AR and 31.7% moderate to severe with a mean regurgitant fraction of 55.3 and mean LVEF of 53.8%. The majority, 91.1%, of procedures were carried out under general anaesthesia with a large valve implanted in 57.2% of cases.
Overall procedural success rates were high, 92.8%, with no in-procedural death, annular rupture or coronary obstruction. Device embolization occurred in 4 cases with one ascending aorta dissection.
- The primary safety endpoint at 30-days occurred in 26.7%, meeting the non-inferiority criteria for the primary endpoint compared to the prespecified performance goal, p<0.0001
- The primary efficacy endpoint (all-cause mortality) occurred in 7.8% at one year, meeting the non-inferiority criteria for the primary efficacy endpoint, p<0.0001
- Pacemaker implants reduced throughout the trial from 30% in the first 60 patients to 14% in the last 60 patients
- Quality of life as assessed by the KCCQ-OS improved from 55.8 at baseline to 77..6 at one year, p<0.0001

Critical reading and the relevance for clinical practice
The results of this study show that in high surgical risk patients with ≥ grade 3 aortic regurgitation, TAVI using the Trilogy transcatheter heart valve met the primary safety endpoint at 30 days and efficacy endpoint at one year compared to pre-specified performance goals. New pacemaker insertion rates occurred in almost one-third of patients in the initial stage of the study but were reduced to 14% at the time of enrolment of the final 60 patients.
The investigators hypothesised that this reduction in PPM rates was related to changes in insertion technique of the valve, placing the locators above the nadir of the native valve cusps along with a reduction in oversizing of the valve. Whilst the rates of PPM implantation are higher than those seen with current TAVI implantation patients undergoing surgical AVR for pure AR have higher pacemaker rates of approximately 12% compared to those undergoing SAVR for AS (1). Therefore whilst there may be opportunities to lower the overall pacemaker rates with this valve it may not reach that of TAVI for AS.
At baseline, the LV impairment and LV mass volumes were relatively low however treatment of AR led to statistically significant reduction in both LVESD and LVESV as assessed by echocardiogram as well as a reduction in LV mass and LV mass index. Given that these patients were symptomatic from their AR close attention needs to be paid to accurate assessment of LV function and volumes to ensure that patients are not denied timely intervention for AR. Rates of paravalvular leak were low with 98.8% assessed by the core lab as having none or mild at 30 days and 100% none or mild at one year follow-up. From a clinical point of view, 90% of patients were NYHA class I or II at 30 days which persisted to 1 year follow-up. Objective assessment of quality of life showed a more than 20 point increase in the KCCQ-OS score at one-year follow-up.
These results are in keeping with the results of real-world consecutive patients with severe AR treated with Trilogy in Germany, where the valve has received a CE mark, and reported at EuroPCR 2022 by Tamm (2). Different to the data reported by Tamm the majority of patients in this study (91.1%) had general anaesthesia for their procedure whilst 82% of cases in the real-world data were performed with conscious sedation. As with all studies, a number of limitations must be considered, all cases were reviewed by a case review board to ensure their suitability for inclusion in the trial with over half of the screened patients failing to meet inclusion criteria. Current guidelines suggest that TAVI can be considered in selected patients in experienced centres (3) and this data further adds to the evidence supporting this recommendation. The longer-term outcomes of the Trilogy valve are as yet unknown however the follow-up of this cohort will extend to five years. The next phase in the evolution of this valve will be assessing its performance compared to SAVR in low and intermediate-surgical risk patients which will require a randomised control trial.
References
- Matthews IG, Fazal IA, Bates MGD, Turley AJ. In patients undergoing aortic valve replacement, what factors predict the requirement for permanent pacemaker implantation? Interact Cardiovasc Thorac Surg. 2011 Mar;12(3):475–9.
- PCR. EuroPCR 2022 Hotlines / Late-Breaking Trials in TAVI: TRANSIT-PPM, LYTEN, SCOPE 2, and more! [Internet]. [cited 2023 Oct 25]. Available from: https://www.pcronline.com/Cases-resources-images/Resources/Course-videos-slides/2022/EuroPCR-2022-Hotlines-Late-Breaking-Trials-in-TAVI-TRANSIT-PPM-LYTEN-SCOPE-2-and-more
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022 Feb 12;43(7):561–632.




No comments yet!