One- versus three-month DAPT after everolimus-eluting stent implantation in diabetic patients at high-bleeding risk: results from the XIENCE Short DAPT programme
Selected in EuroIntervention Journal by N. Ryan
This analysis from the XIENCE Short DAPT programme compared the safety and efficacy of one-month versus three-month DAPT in high-bleeding risk patients with and without diabetes mellitus (DM) following PCI with everolimus eluting stents (EES).
References
Authors
Oliva Angelo, Angiolillo Dominick, Valgimigli Marco, Cao Davide, Sartori Samantha, Bangalore Sripal, Bhatt Deepak, Campo Gianluca, Chehab Bassem, Choi James, de la Torre Hernandez Jose, Feng Yihan, Ge Junbo, Gitto Mauro, Hermiller James, Krucoff Mitchell, Kunadian Vijay, Makkar Raj, Maksoud Aziz, Neumann Franz-Josef, Picon Hector, Saito Shigeru, Sardella Gennaro, Thiele Holger, Toelg Ralph, Varenne Olivier, Vogel Birgit, Vranckx Pascal, Windecker Stephan, Mehran Roxana
Reference
DOI: 10.4244/EIJ-D-24-00897
Published
Jun 16, 2025
Link
Read the abstractReviewer
Latest contributions
Balloon lithotripsy added to conventional preparation before stent implantation in severely calcified coronary lesions Impact of pullback pressure gradient on clinical outcomes after percutaneous coronary interventions Validation of intravascular ultrasound-defined optimal stent expansion criteria for favorable 1-year clinical outcomesMy Comment

Designed by: Nicola Ryan
Source: PCRonline.com
Why this study – the rationale/objective?
Dual antiplatelet therapy aims to reduce ischaemic risk post PCI, but comes with a concomitant increased risk of bleeding. Patients with diabetes mellitus are at increased risk of ischaemic events, with diabetes mellitus commonly used a criteria to consider prolonged or intensified DAPT. Given the known morbidity and mortality associated with bleeding, shortened DAPT is proposed to reduce the bleeding risk in high-risk patients.
Prior studies have established that PCI with DES and one month of DAPT is a safe and efficacious strategy in all-comer high bleeding risk patients1, however, patients with both diabetes mellitus and high bleeding risk characteristics present a clinical dilemma with the most appropriate antiplatelet strategy not well defined. The optimal duration of short DAPT in patients with diabetes mellitus is unknown, therefore this analysis of the XIENCE Short DAPT programme compared the clinical outcomes of one versus three months DAPT in HBR patients with and without DM.
How was it executed? The methodology
The XIENCE Short DAPT programme includes three prospective multicentre registries; Xience 90 (3-month DAPT), Xience 28 USA and Xience 28 Global (1-month DAPT) enrolling patients undergoing PCI with fluoropolymer-based cobalt-chromium everolimus eluting stents for acute or chronic coronary syndrome with at least one high-bleeding risk criteria from July 2017 to February 2020.
Patients were treated with aspirin and a P2Y12 inhibitor, preferentially clopidogrel, and were assessed at one month and three months respectively (Xience 28 and Xience 90) to ensure they remained eligible for DAPT discontinuation (no adverse events and compliant with therapy). This analysis included the Xience 28 populations with patients who were event-free at one month in the Xience 90 population selected to match the Xience 28 population.
- The primary outcome was a composite of all cause death or MI between 1 and 12 months post index PCI
- The key secondary endpoint was BARC Type 2 to 5 bleeding
- Other secondary endpoints included target lesion failure (a composite of cardiovascular death, target vessel MI or target lesion revascularisation), the individual components of the composite endpoints, stroke, definite or probable stent thrombosis and BARC Type 3 to 5 bleeding.
The effects of one- versus three-month DAPT on outcomes was evaluated according to diabetes mellitus status.
What is the main result?

Designed by: Nicola Ryan
Source: PCRonline.com
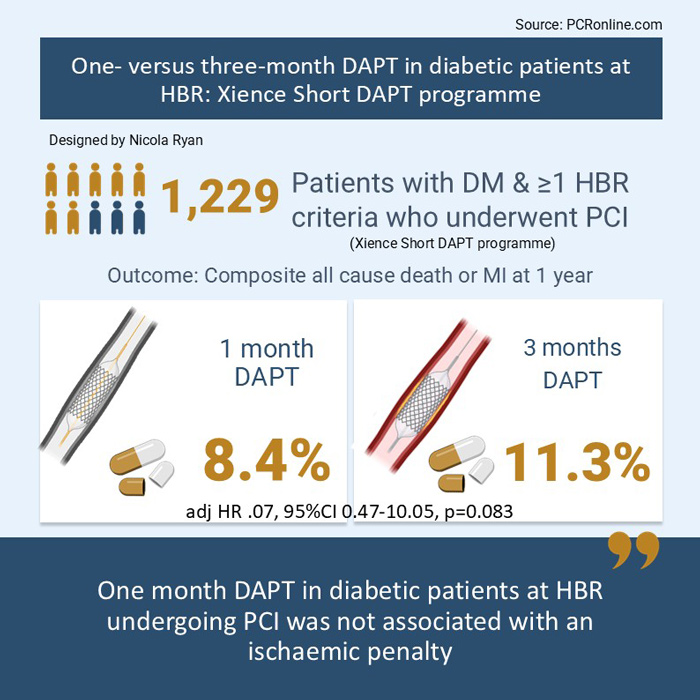
Of the 3,352 eligible patients, 1,299 patients had diabetes mellitus (512 and 787 received one- and three-month DAPT respectively) and 2,152 did not have DM (870 and 1,283 received one- and three-month DAPT respectively). Patients with DM were younger (mean age 73.4 vs 76.8 years), less commonly white, had more traditional CVRF and multivessel disease than those without.
- At one-year follow-up, patients with DM had a higher incidence of the composite of all-cause death and MI compared to those without (10.1 % vs. 6.6 % HR 1.51, 95 % CI 1.18-1.94, p = 0.001), with no significant differences in bleeding.
- There was no significant difference in the composite of all-cause death and MI in diabetic patients treated with 1- vs 3-month DAPT (8.4 % vs 11.3 %, adjHR 0.70, 95 % 0.47—10.05, p = 0.083).
- In diabetic patients, there was lower rates of MI (3.3 % vs 6.8 %, adjHR 0.46, 95 % CI 0.26-0.84, p = 0.011) and TLF (4.8 % vs 8.6 %, adjHR 0.54, 95% CI 0.33-0.89) in the group treated with one-month DAPT compared to three-month DAPT.
- Overall, NACE was lower in the one-month DAPT group in patients with DM (11 % vs 15.4 %, adjHR 0.64, 95 % CI 0.46-0.9, p = 0.01).
- There were no significant differences in BARC 2-5 (8.1 % vs 10.4 %, adjHR 0.67, 95 % CI 0.45-1.01, p = 0.057) or BARC 3-5 (4.5 % vs 4.7 %, adjHR 0.77, 95 % CI 0.44-1.37, p = 0.381) bleeding between one- and three-month DAPT groups in diabetic patients.
Critical reading and the relevance for clinical practice:
The results of this analysis show that, in the overall XIENCE Short DAPT population, patients with diabetes had an increased ischaemic and similar bleeding risk compared to those without diabetes. In the diabetic population, there was a similar rate of the primary composite endpoint, all-cause mortality and MI, as well as the key secondary endpoint, BARC 2-5 bleeding, in the one-month DAPT group compared to three-month DAPT group. In the diabetic population, overall NACE was lower in the one-month DAPT group predominantly driven by lower MI and TLR in patients as well as a numerically but not statistically lower rate of bleeding in the one-month group.
The increased ischaemic risk in patients with diabetes has been well established, however, the HBR population is unique, particularly given that a bleeding event may precipitate an ischaemic event. Therefore, the proposed benefits of prolonged DAPT in patients with diabetes may not be applicable in the HBR population. A pre-specified analysis of the MASTER DAPT trial did not demonstrate any differences in bleeding or ischaemic events in patients with or without diabetes treated with standard (≥ 3 months) or abbreviated (1 month) DAPT2. Different to the current analysis, three quarters of patients in the MASTER DAPT trial continued with P2Y12 monotherapy as opposed to aspirin monotherapy.
In the XIENCE short DAPT population, patients with DM had more extensive coronary artery disease and twice the risk of MI at one year compared to patients without diabetes. Interestingly, in the diabetic group, the risk of MI was significantly higher in the three-month DAPT group, with numerically but not statistically higher bleeding in this group compared to one-month DAPT. The increased rate of MI in the three-month DAPT group deserves some consideration. Given that the study was not randomised to assess the diabetic population, nor powered to assess the individual endpoints of the primary endpoint, this may simply be play of chance. Furthermore, patients from the Xience 90 trial were retrospectively analysed to confirm their eligibility for inclusion at one month; despite adjustment, residual confounders may persist. Importantly, approx. 14 % of patients assigned to three-month DAPT remained on DAPT at one year; it is unclear whether this was due to an ischaemic event leading to prolongation of DAPT or physician or patient preference. Moreover, it is unclear if the numerically increased bleeding events in the three-month DAPT arm may be attributed to the prolongation of DAPT beyond the randomised three months. Given that a bleeding event may precipitate an ischaemic event, it would be of interest to understand the total and recurrent events in this population.
This analysis is of interest to the community as high-bleeding risk patients with diabetes mellitus are frequently encountered in clinical practice. Whilst the increased ischaemic events in the DM population need to be carefully considered when abbreviating DAPT, extending DAPT to three months compared to one month does not confer an ischaemic benefit, whilst there was numerically reduced bleeding with one-month DAPT, this did not reach statistical significance.
Overall, the trial can be interpreted as showing that, in a relatively low-risk population with diabetes and HBR, criteria one-month DAPT does not carry an ischaemic penalty compared to three-month DAPT, with lower rates of NACE and numerically but not statistically lower bleeding events. Therefore, in daily clinical practice, one-month of DAPT in HBR patients with a similar profile to the XIENCE Short DAPT population, i.e. predominantly chronic coronary syndrome with low complexity coronary artery disease, including those with DM may be an appropriate strategy. Importantly, this analysis only includes patients who were event-free and compliant with medications at one month review; therefore this is a strategy that can only be applied following clinical review at one month. As always, decisions with regard to ischaemic and bleeding risk, along with choice of antiplatelet agent, need to be carefully assessed and individualised to each patient.
References
- Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrié D, Naber C, et al. Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. N Engl J Med. 2015 Nov 19;373(21):2038–47.
- Roffi M, Landi A, Heg D, Frigoli E, Chalkou K, Chevalier B, et al. Abbreviated or Standard Antiplatelet Therapy After PCI in Diabetic Patients at High Bleeding Risk. JACC: Cardiovascular Interventions. 2024 Nov 25;17(22):2664–77.
- Smits PC, Frigoli E, Vranckx P, Ozaki Y, Morice MC, Chevalier B, et al. Abbreviated Antiplatelet Therapy After Coronary Stenting in Patients With Myocardial Infarction at High Bleeding Risk. Journal of the American College of Cardiology. 2022 Sep 27;80(13):1220–37.




No comments yet!