Five-year clinical and echocardiographic outcomes from the PARTNER 3 Low-Risk randomized trial
Reported from TCT 2023
Alex Sticchi provides his take on the 5-year outcomes from PARTNER 3 Low Risk which were presented by Martin B. Leon at TCT Congress 2023.
Why this study – the rationale/objective?
Transcatheter aortic valve implantation (TAVI) is one of the most successful and spread procedures for treating symptomatic patients with severe aortic stenosis. After the favourable outcomes shown in the comparison with surgical aortic valve replacement (SAVR) in patients at intermediate or high risk for mortality 30 days after surgery, the current studies are involving younger patients at low risk. In two trials comparing TAVI to surgery in this last scenario, the percutaneous approach showed to be non-inferior or superior to surgery at 2 or 3 years. The Placement of Aortic Transcatheter Valves (PARTNER) 3 trial [1] reported a significantly lower rate of events for the the composite end point of death, stroke, or rehospitalization at 1 and 2 years with TAVI compared to SAVR.

How was it executed - the methodology?
PARTNER 3 is a multicenter, randomized trial (1:1) comparing TAVI using the balloon-expandable valve SAPIEN 3 (Edwards Lifesciences) with SAVR in patients with symptomatic severe aortic stenosis at low-risk (STS-PROM <4%) on clinical and anatomical assessment through heart-team evaluation.
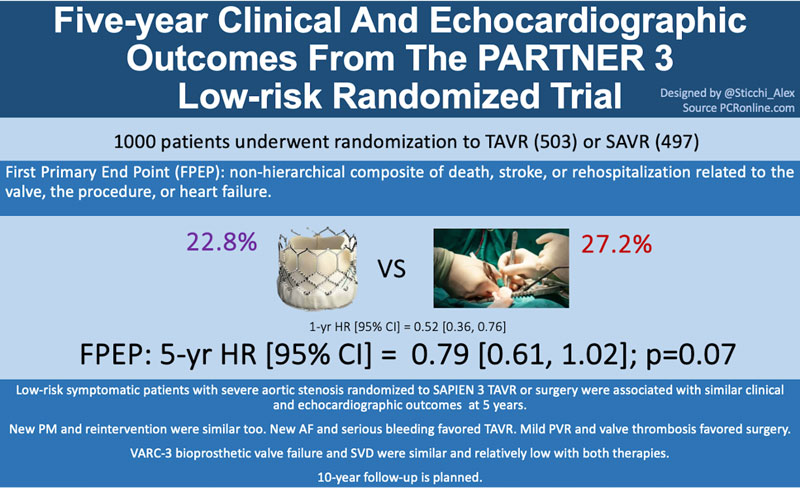
The first primary endpoint was a non-hierarchical composite of death from any cause, stroke, or rehospitalization. The second primary endpoint was a hierarchal composite of death from any cause, disabling stroke, non-disabling stroke, and the number of rehospitalization days. A time-to-event analysis from baseline to 1 year, to 1 to 5 years (landmark analysis), and baseline to 5 years were also performed, calculating hazard ratios (HR) and 95% confidence intervals (CI) for the clinical endpoints.
What is the main result?
The 5-year follow-up was reached for 94.6% of patients undergone TAVI and 88.3% of the group with SAVR.
The first primary endpoint showed similar event rates for the two groups with 22.8% in the TAVR group and 27.2% in the surgery group at the Kaplan-Meier analysis (−4.3 percentage points; 95% CI −9.9 to 1.3; P = 0.07; HR, 0.79; 95% CI, 0.61 to 1.02). This result was consistent in the subgroup analysis, and the Kaplan-Meier estimates of years 1 to 5 were 15.7% in the TAVI group and 13.7% in the surgery group (HR, 1.17; 95% CI, 0.81 to 1.70). The second primary endpoint assessed via the Win Ratio method showed a total win score of 22.1% for the TAVI and 19.0% for the SAVR with a ratio of 1.17 (95% CI, 0.90 to 1.51; P = 0.25).
The mortality at 5 years was 10.2% versus (vs) 9.0% in the TAVI and the surgery group, respectively. Numerically, the study reported at 5 years 48 deaths in the TAVI group (26 from cardiovascular causes and 22 from non-cardiovascular causes) vs 34 in the surgery group (21 from cardiovascular causes and 13 from non-cardiovascular causes).
Moreover, no differences occurred in the new permanent pacemaker rate (13.5% vs 10.4%) and regarding stroke (5.8% and 6.4%, (HR, 0.87; 95% CI, 0.51 to 1.48.) respectively for TAVR and SAVR).
From the echocardiographic point of view, the aortic-valve gradient at 5 years was 12.8±6.5 mmHg in the TAVR group and 11.7±5.6 mmHg in the SAVR group. Furthermore, no differences occurred in the analysis of the primary endpoint according to the valve size (5-yr HR [95% CI] = 0.81 [0.51, 1.29]; P = 0.62) and for the valve hemodynamics regarding aortic valve area (P = 0.90).
Finally, bioprosthetic-valve failure (3.8% vs 3.3%, HR [95% CI] = 0.86 [0.42, 1.77], P = 0.69) and the need for reintervention (2.2% vs 2.6%) resulted similar at 5 years in the two groups.
Critical reading and the relevance for clinical practice
The data deriving from the 5-year analysis are definitely very interesting for the field and mitigate the enthusiasm of the one-year report where the rates were in favour of the TAVI choice.
This is partially expected due to the immediate winning of the TAVI with the percutaneous approach, but this does not make the study less promising, and we need to congratulate the authors for the consistent results. In particular, I would like to stress the mortality data, which includes both cardiovascular and non-cardiovascular deaths. It seems the adjudication of cardiovascular death was very strict in PARTNER 3, and they reported a 5.5% rate in the TAVI arm vs 5.1% in the SAVR group at 5 years, while the non-cardiovascular deaths were numerically higher in the TAVI compared to the SAVR arm (22 vs 13 deaths, respectively). The mortality curves get close at 2-3 years, and this is not an issue but something to consider in the future long-term follow-up.
Clearly, TAVI choice in the PARTNER 3 low-risk trial is non-inferior to SAVR, and even if the differences in the primary endpoint rate are attenuated (from 7.1% to 4.3%), we have several endpoints similar (new PM and reintervention), or in favour to TAVI (new AF and severe bleeding).
Mild or greater residual aortic regurgitation (20.8% vs 3.2%) and valve thrombosis (2.5% vs 0.2%) continue favouring SAVR, but we have to see future technological and therapeutic management evolution.
So, these are not final results and the apparent inversion of the trend could ring a bell in the longer term, probably more for the intra-annular position than the transcatheter approach itself, considering the differently oriented data of the other low-risk trial with a self-expandable supra-annular valve [2]. This could not be true considering the elegant work of Okuno et al. from the Bern group with similar clinical outcomes between the two devices in the challenging scenario of the small annuli [3]. Moreover, the two low-risk trials are significantly different in the methodology and population enrolled, so it is probably unfair to compare them.
We need more data at longer term (10 years at least), but technological evolution, procedural technique and pharmacologic therapy will help the field improve more in the following years. And I trust the surgery field will do the same.
As Heart-Team and scientific researchers, the game for the aortic treatment life-long management is still completely open.
References:
- Mack MJ, et al. Transcatheter aortic-valve replacement in low-risk patients at five years. New Engl J Med. 2023; October 24, 2023. DOI: 10.1056/NEJMoa2307447.
- Forest JK, et al. 4-year outcomes of patients with aortic stenosis I the Evolut Low-Risk Trial. J Am Coll Cardiol. Oct 24, 2023. Epublished DOI: 10.1016/j.jacc.2023.09.813
- Okuno T, et al. 5-Year Outcomes With Self-Expanding vs Balloon-Expandable Transcatheter Aortic Valve Replacement in Patients With Small Annuli. JACC Cardiovasc Interv. 2023 Feb 27;16(4):429-440. DOI: 10.1016/j.jcin.2022.11.032.




No comments yet!