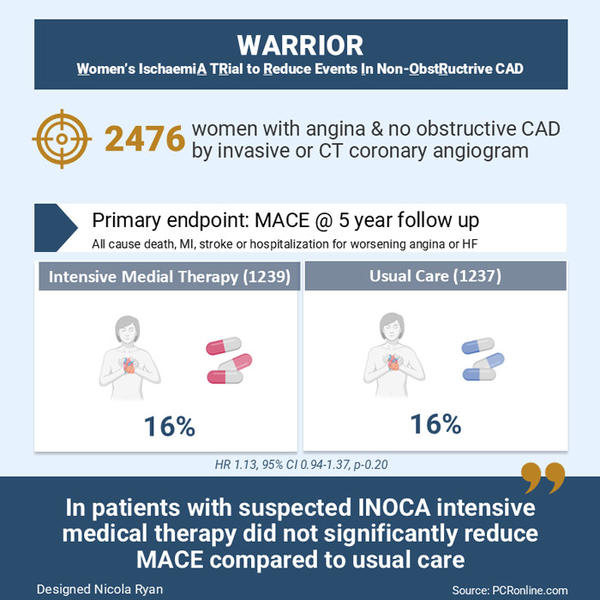
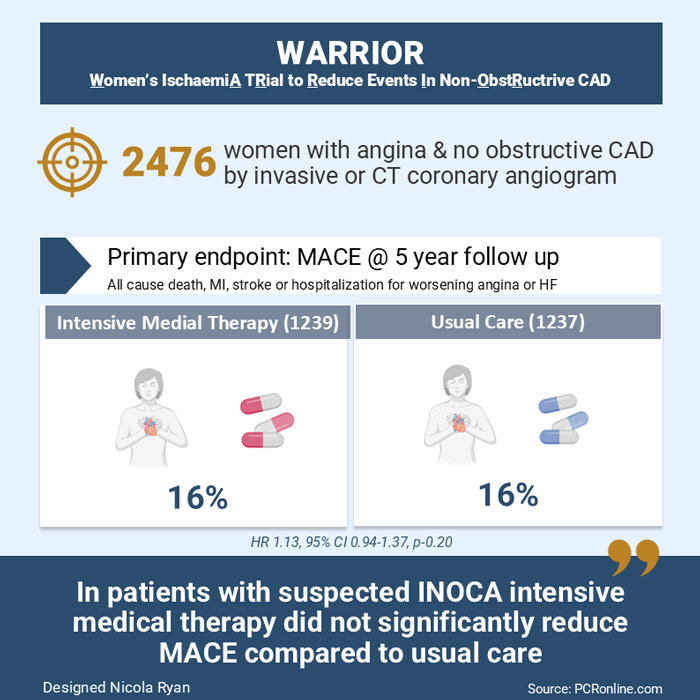
Primary and secondary outcomes of the Women's Ischemia Trial to reduce events in non-obstructive coronary artery disease (WARRIOR)
Reported from ACC.25
Nicola Ryan provides her take on the WARRIOR trial which was presented by Eileen Handberg at ACC.25 in Chicago.
The manuscript for this trial has not been published and all assumptions are based upon the presentation.
Designed by Nicola Ryan. Source: PCRonline.com
Why This Study – Rationale/Objectives
Up to 50% of patients referred for coronary angiography due to a clinical suspicion of angina have no obstructive coronary artery disease. This subgroup includes patients with Ischaemia with Non-Obstructed Coronary Arteries (INOCA) who have a poorer outcome in terms of adverse events, quality of life and increased healthcare costs. The Warrior trial aimed to investigate if intensive medical management improved outcomes in patients with suspected INOCA compared to usual care.
Methodology
This study was a prospective, multicenter, randomized, blinded outcome trial. Women were eligible for inclusion if they presented with clinically stable angina/angina equivalent and had no obstructive coronary artery disease (>50% stenosis) on either invasive coronary angiography or coronary CT angiogram. Key exclusion criteria were cardiomyopathies, significant valvular or uncorrected congenital heart disease, HIV, hepatitis C, renal impairment eGFR<30, liver disease or an expected survival < 2 years. Patients were randomized to intensive medical therapy with high-intensity statin or PCSK9i, ACEi or ARB and low-dose aspirin. The control group received medical therapy at the discretion of the treating physician.
The primary outcome was
- MACE: All-cause death, MI, stroke or hospitalization for worsening angina or heart failure.
Secondary outcomes included
- Quality of life, time to return to work, healthcare utilization, angina, CV death
Main Results
A total of 2476 women with clinically stable angina and no obstructive CAD were included, 1239 in the intensive medical therapy group (675 underwent invasive angio and 564 CCTA) and 1237 in the usual care group (678 underwent invasive angio and 559 CCTA).
The mean age was 64 years with a high prevalence of diabetes and hypertension. The baseline mean SAQ7 score was 69.7 and 71.6 in the intensive and usual care groups respectively suggesting a low overall burden of angina. Medication prescription was similar between groups with 70% on a statin, 53.1 and 48.2% on an ACEi or ARB and 62.9 and 58.5% on aspirin in the intensive and usual care groups respectively. There were similar rates of prescription of beta-blockers and calcium channel blockers.
- There was no difference in the primary outcome between groups at 5-year follow-up (HR 1.13, 95% CI 0.94-1.37, p=0.20)
- There were no significant differences in the components of the primary endpoint between groups with low rates of CV death (4 vs 5), non-fatal MI (14 vs. 14) in the intensive versus usual care respectively
- The majority of the primary endpoint events were hospitalization for chest pain (3.05 vs 298) in the intensive versus usual care strategy.
Critical Analysis and Clinical Relevance
This randomised control trial demonstrated no difference in outcomes in patients with suspected INOCA treated with intensive medical therapy versus usual care. This is an important topic to study as no obstructive coronary artery disease is a common finding at invasive angiography. A major limitation of the trial is that the prescription of statin, ACEi/ARB and low-dose aspirin were similar between groups therefore it is impossible to draw meaningful conclusions from the trial. Moreover, the trial was underpowered for the primary endpoint.
A further important consideration is that the WARRIOR trial includes patients with suspected INOCA rather than confirmed INOCA. INOCA is an umbrella term for coronary microvascular dysfunction and vasospastic angina which are different entities. Whilst some of the principles of treatment are similar the CorMicA trial demonstrated that stratified medical therapy based on the underlying substrate leads to improved control of angina and quality of life. Furthermore given that formal assessments for CMD and VSA were not carried out it is reasonable to assume that a proportion of the patients included in the trial have non-cardiac chest pain. This subgroup may have benefited from further investigation of alternative causes for chest pain had cardiac chest pain been excluded.
Finally, the majority of the primary endpoint was driven by hospitalizations for chest pain, this is a subjective outcome and given that patients did not have a definitive diagnosis of CMD, VSA or non-cardiac chest pain there may have been increased healthcare-seeking behaviour from the patients as well as an increased likelihood to admit from the physicians. The CorMica trial demonstrated that a definitive diagnosis led to decreased healthcare utilization.
Overall this study raises awareness of INOCA as a diagnosis however future trials should focus on stratified medical therapy based on the underlying diagnosis. Invasive coronary function testing carries a 1B recommendation in the current ESC guidelines for chronic coronary syndrome, given it is a relatively simple additional investigation consideration should be given to adequately assessing all patients with no obstructive CAD to ensure they receive optimal medical care.
References
- Ford TJ, Stanley B, Good R, et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J Am Coll Cardiol. 2018;72(23 Pt A):2841-2855
- Heggie, R.et al. Stratified medicine using invasive coronary function testing in angina: A cost-effectiveness analysis of the British Heart Foundation CorMicA trial International Journal of Cardiology, Volume 337, 44 - 51




No comments yet!