Impact of post-stenting fractional flow reserve on long-term clinical outcomes - The FFR-SEARCH Study
Selected in Circulation: Cardiovascular Interventions by M. Pighi
The objective of this analysis was to prospectively investigate the clinical impact of post-PCI FFR values on long-term clinical outcomes.
References
Authors
Roberto Diletti, Kaneshka Masdjedi, Joost Daemen, Laurens J.C. van Zandvoort, Tara Neleman, Jeroen Wilschut, Wijnand K. Den Dekker, Rutger J. van Bommel, Miguel Lemmert, Isabella Kardys, Paul Cummins, Peter de Jaegere, Felix Zijlstra, Nicolas M. Van Mieghem
Reference
10.1161/CIRCINTERVENTIONS.120.009681
Published
March 2021
Link
Read the abstractReviewer
My Comment
Why this study? – the rationale/objective
According to the updated international revascularization guidelines, fractional flow reserve (FFR) is proposed as the standard of care for ischemia detection in guiding percutaneous coronary intervention (PCI) of intermediate coronary stenosis.
To date, post-PCI FFR evaluation has been poorly investigated, with few retrospective analyses showing a relationship between post-PCI FFR and clinical outcomes, with a cut-off of FFR < 0.90 hypothesized as defining a suboptimal result after FAME 1 and FAME 2 trials.
The objective of this analysis was to prospectively investigate the clinical impact of post-PCI FFR values on long-term clinical outcomes.
How was it executed? – the methodology
- This prospective, single-center, open-label, all-comers study (FFR-SEARCH study) included 959 patients undergoing PCI with stent implantation.
- FFR was evaluated post-stenting, when the operator considered the angiographic result acceptable and final. No further interventions were allowed after measurement, regardless of the final FFR value.
- Included patients were all comers with stable coronary artery disease and acute coronary syndromes (STEMI and NSTEMI).
- The comparison on long-term outcomes was performed using a post-PCI cut-off of 0.90. A per-patient analysis and a per-vessel analysis were performed.
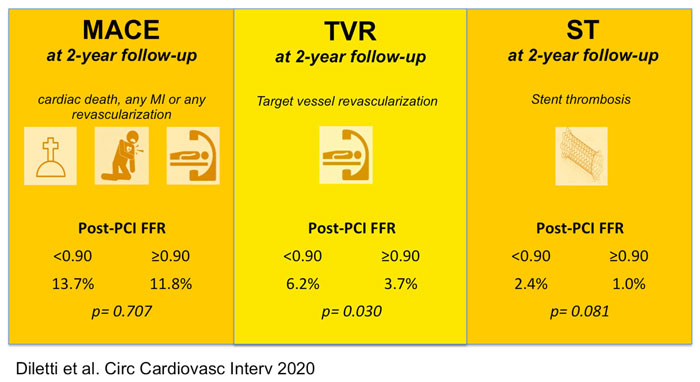
- The primary endpoint was MACE incidence, including cardiac death, spontaneous MI, and any revascularization.
- The secondary endpoints included each separate component of the primary endpoint, TVRs, target vessel MI and scaffold thrombosis.
What is the main result?
- Post-stenting assessment is safe, feasible, and easy to perform, with no FFR microcatheter use-related complications observed.
- Despite optimal angiographic results, an impairment of post-PCI FFR (< 0.90) was common (37.8 % of patients).
- Post-PCI FFR < 0.90 was not associated with a significant increase in overall MACE (HR 1.08 [95 % CI, 0.73–1.60]; P = 0.707).
- Conversely, it was associated with:
- a significant increase in TVR (HR, 1.91 [95 % CI, 1.06–3.44]; P = 0.030)
- and a trend towards a higher rate of stent thrombosis (HR, 2.89 [95 % CI, 0.88–9.48]; P = 0.081) on a vessel level analysis during 2-year follow-up.
Critical reading and the relevance for clinical practice:
This report represents the first prospective study evaluating the impact of microcatheter-based post-stenting FFR on long-term clinical outcomes.
The results showed that post-PCI FFR is safe and feasible and that a large proportion of real-world PCI - the result of which was deemed as optimal from an angiographic standpoint - presented a suboptimal physiologic result.
Furthermore, even if FFR values did not impact significantly on MACE, a post-PCI FFR < 0.90 was related to a substantially higher incidence of TVR and a numerical increase of definite scaffold thrombosis at follow-up.
These data are in line with previous retrospective studies that showed a moderate impact of suboptimal post-PCI FFR on hard clinical endpoints and a more relevant impact on vessel-specific endpoints.
Thus, from a post hoc analysis of FAME 1 and FAME 2 trials, the upper tertile of post-PCI FFR showed a significantly higher overall vessel-oriented composite endpoint (VOCE) and TVR compared to the lower ones1. Similar findings have been provided, based on quantitative flow reserve (QFR), a novel angiography-based adenosine and pressure-wire free index.
Indeed, a retrospective analysis of the SYNTAX II trial showed that a moderate diagnostic performance of post-PCI QFR in predicting 2-year VOCE (AUC 0.702; 95 % confidence interval: 0.633 to 0.772), with a significantly higher incidence of 2-year VOCE in vessels with post-PCI QFR< 0.91 (12.0 % vs. 3.7 %; HR: 3.37; 95 % CI: 1.91 to 5.97; p < 0.001)2.
Similarly, Biscaglia et al., in the prospective HAWKEYE study, showed that lower values of QFR after complete and successful revascularization predicted subsequent adverse events. In particular, the analysis of 602 patients demonstrated that, after correction for potential confounding factors, post-PCI QFR < 0.90 was associated with a 3-fold increase in risk for VOCE (HR: 2.91; 95 % CI: 1.63 to 5.19; p < 0.001)3.
Although previous retrospective analyses showed a correlation between lower post-stent FFR and adverse outcomes, the penetration of physiology post-PCI assessment in clinical practice is still low.
According to the recent ERIS study, post-PCI FFR was evaluated in less than 10 % of lesions with the pre-PCI evaluation4. These data are consistent with the heterogeneous adoption of physiology in the real-world setting, with large areas performing less than 15 % of eligible procedures with the physiological assessment5.
One of the main reasons that may limit post-PCI physiology penetration into the catheterization laboratory routine is the lack of randomized prospective trials and evidence on the best management of post-PCI suboptimal functional results. The pressure pullback seems to represent a key tool in defining the underlying problem and consequent intervention.
According to the DEFINE PCI study, a suboptimal iFR result was detected in 24 % of patients, with 18.4 % with diffuse disease and 81.6 % with a focal disease, 38.4 % within the stented segment, and 61.6 % proximal or distal to the stent6.
In the case of diffuse disease, it is unlikely that further interventions would provide improvement in the physiological result. Indeed, the use of long stents could increase the risk for stent-restenosis, while it is well known that stent underexpansion and edge dissections have a detrimental impact on the long-term outcomes7.
To conclude, the results of the current work by Diletti et al., representing the first prospective trial with the aim to post-PCI FFR, confirm a higher incidence of TVR in lesions with FFR < 0.90. This study paves the way for future studies supporting the use of extensive physiological guidance in percutaneous interventions.
Of note, ongoing trials such as FFR-REACT (FFR-Guided PCI Optimization Directed by High-Definition IVUS Versus Standard of Care) and TARGET-FFR (An Evaluation of a Physiology-Guided PCI Optimisation Strategy) may provide further evidence to the integrated use of physiology and intracoronary imaging in planning and optimization of PCI, with a potential role in reducing long-term clinical outcomes and ischemic symptoms after successful PCI.
Non-hyperemic pressure indexes and angiography-derived ones (iFR, QFR, FFRangio, and vFFR) may offer additional information to define the exact location and mechanism of suboptimal post-PCI physiology results, and, thus, leading to a patient-tailored treatment (i.e. conservative medical therapy or additional PCI).
References
- Piroth Z, Toth GG, Tonino PAL, Barbato E, Aghlmandi S, Curzen N, Rioufol G, Pijls NHJ, Fearon WF, Jüni P, De Bruyne B. Prognostic Value of Fractional Flow Reserve Measured Immediately After Drug-Eluting Stent Implantation. Circ Cardiovasc Interv. 2017;10.
- Kogame N, Takahashi K, Tomaniak M, Chichareon P, Modolo R, Chang CC, Komiyama H, Katagiri Y, Asano T, Stables R, Fath-Ordoubadi F, Walsh S, Sabaté M, Davies JE, Piek JJ, van Geuns RJ, Reiber JHC, Banning AP, Escaned J, Farooq V, Serruys PW, Onuma Y. Clinical Implication of Quantitative Flow Ratio After Percutaneous Coronary Intervention for 3-Vessel Disease. JACC Cardiovasc Interv. 2019;12:2064–75.
- Biscaglia S, Tebaldi M, Brugaletta S, Cerrato E, Erriquez A, Passarini G, Ielasi A, Spitaleri G, Di Girolamo D, Mezzapelle G, Geraci S, Manfrini M, Pavasini R, Barbato E, Campo G. Prognostic Value of QFR Measured Immediately After Successful Stent Implantation: The International Multicenter Prospective HAWKEYE Study. JACC Cardiovasc Interv. 2019;12:2079–88.
- Tebaldi M, Biscaglia S, Fineschi M, Musumeci G, Marchese A, Leone AM, Rossi ML, Stefanini G, Maione A, Menozzi A, Tarantino F, Lodolini V, Gallo F, Barbato E, Tarantini G, Campo G. Evolving routine standards in invasive hemodynamic assessment of coronary stenosis: The nationwide Italian SICI-GISE cross-sectional ERIS study. JACC Cardiovasc Interv. 2018;11:1482–91.
- Hennigan B, Oldroyd KG, Berry C, Johnson N, McClure J, McCartney P, McEntegart MB, Eteiba H, Petrie MC, Rocchiccioli P, Good R, Lindsay MM, Hood S, Watkins S. Discordance between Resting and Hyperemic Indices of Coronary Stenosis Severity: The VERIFY 2 Study (A Comparative Study of Resting Coronary Pressure Gradient, Instantaneous Wave-Free Ratio and Fractional Flow Reserve in an Unselected Population Referred . Circ Cardiovasc Interv. 2016;9:1–10.
- Jeremias A, Davies JE, Maehara A, Matsumura M, Schneider J, Tang K, Talwar S, Marques K, Shammas NW, Gruberg L, Seto A, Samady H, Sharp A, Ali ZA, Mintz G, Patel M, Stone GW. Blinded Physiological Assessment of Residual Ischemia After Successful Angiographic Percutaneous Coronary Intervention: The DEFINE PCI Study. JACC Cardiovasc Interv. 2019;12:1991–2001.
- Biscaglia S, Uretsky B, Barbato E, Collet C, Onuma Y, Jeremias A, Tebaldi M, Hakeem A, Kogame N, Sonck J, Escaned J, Serruys PW, Stone GW, Campo G. Invasive Coronary Physiology After Stent Implantation. JACC Cardiovasc Interv. 2021;14:237–46.






No comments yet!