08 Nov 2022
Aspirin vs Clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention: the HOST-EXAM extended study
Selected in Circulation by N. Ryan
The HOST-EXAM trial1 was a prospective, randomised, open-label trial investigating clopidogrel monotherapy versus aspirin therapy in patients undergoing PCI with DES who had completed 6-18 months of DAPT with no ischaemic or bleeding complications. At 24 months follow-up, clopidogrel monotherapy significantly reduced the risk of the composite endpoint of all-cause death, non-fatal MI, stroke, readmission due to ACS, and BARC 3-5 bleeding. Here the authors report the extended follow-up of the HOST EXAM trial to a median of 5.8 years.
References
Authors
Jeehoon Kang, Kyung Woo Park, Huijin Lee, Doyeon Hwang, Han-Mo Yang, Seung-Woon Rha, Jang-Whan Bae, Nam Ho Lee, Seung Ho Hur, Jung-Kyu Han, Eun-Seok Shin, Bon-Kwon Koo and Hyo-Soo Kim
Reference
Originally published: 7 Nov 2022 | https://doi.org/10.1161/CIRCULATIONAHA.122.062770 | Circulation. 2022;0
Published
7 November 2022
Link
Read the abstract
Reviewer
Latest contributions
Balloon lithotripsy added to conventional preparation before stent implantation in severely calcified coronary lesions Impact of pullback pressure gradient on clinical outcomes after percutaneous coronary interventions Validation of intravascular ultrasound-defined optimal stent expansion criteria for favorable 1-year clinical outcomesMy Comment
PICOT Scheme - Courtesy of Nicola Ryan @NicolaRyanl1, Source: PCRonline.com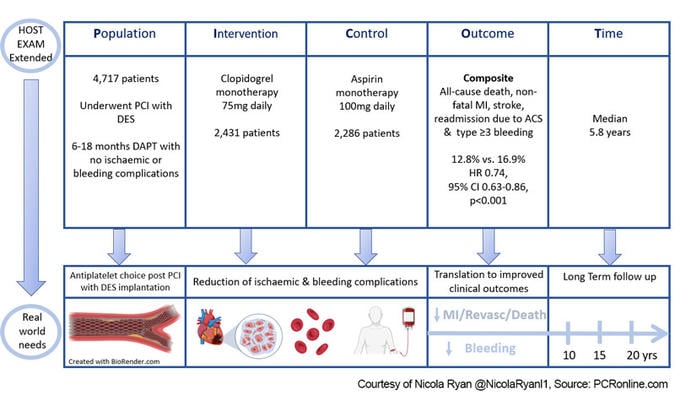
Why this study – the rationale/objective?
Antiplatelet therapy post-PCI aims to reduce ischaemic events; however, comes with the concomitant increased risk of bleeding. Traditionally, following a period of dual antiplatelet therapy, aspirin monotherapy is prescribed lifelong to reduce the ischaemic risk. More recently, trials1,2 have suggested the P2Y12 monotherapy may be preferable in terms of reducing bleeding risk without increasing ischaemic risk.
In the HOST-EXAM trial, at two years, there was a significant reduction in both bleeding and ischaemic events in the clopidogrel arm. Given that antiplatelet therapy is typically prescribed lifelong, it is of interest to understand the longer-term outcomes in this population.
How was it executed? - the methodology
Patients undergoing PCI with a drug-eluting stent who completed 6-18 months of DAPT without any ischaemic or major bleeding complications were eligible for inclusion. Patients were randomised 1:1 to monotherapy with clopidogrel 75 mg daily or aspirin 100 mg daily. After the initial two-year trial follow-up period, the antiplatelet agent was at the discretion of the treating physician.
- The primary outcome was a composite of all-cause death, non-fatal MI, stroke, readmission due to acute coronary syndrome, and BARC type ≥ 3 bleeding.
- The secondary composite endpoints included the thrombotic composite endpoint (cardiac death, non-fatal MI, ischaemic stroke, readmission due to ACS, and definite or probable stent thrombosis) and any bleeding (BARC type ≥ 2).
- Individual components of the primary and secondary composite endpoints were included as secondary endpoints.
What is the main result?
Overall, between March 2014 and May 2018, 5,438 patients were included in the trial. Following exclusion, [9 withdrew consent, 618 used alternative antiplatelet (583 following the 2-year follow-up), 94 lost to follow-up], this analysis included 4,717 patients in the per-protocol population, 2,431 in the clopidogrel arm, and 2,286 in the aspirin arm. Of the patients who were excluded due to antiplatelet change post the original trial period, the majority were due to the prescribing physicians' choice with increased medication discontinuation in the aspirin group (13.5 % vs. 8.0 %, p < 0.001).
- The composite primary endpoint occurred in 12.8 % of the clopidogrel group compared to 16.9 % of the aspirin group at a median follow-up of 5.8 years. (HR 0.74, 95 % CI 0.63-0.86, p < 0.001). Giving an absolute risk reduction of 4.1 % (95 %CI 2.1-6.2 %), NNT 24.
- The thrombotic endpoint was lower in the clopidogrel group compared to the aspirin group. (8.1 % vs. 11.9 % HR 0.66, 95 % CI 0.55-0.79, p < 0.001)
- Similarly, any bleeding was lower with clopidogrel (4.5 % vs 6.1 % HR 1.04, 95 % CI 0.57-0.94, p = 0.016).
- There were no significant differences in all-cause death between the groups (clopidogrel 6.2 % vs aspirin 6.0 % HR 1.04, 95 % CI 0.82-1.31, p = 0.742).
- Landmark analysis showed the benefit of clopidogrel was consistent during both the initial trial period as well as the extended follow-up.
Critical reading and the relevance for clinical practice
The results of this extended follow-up of the HOST-EXAM trial show that the benefit of clopidogrel monotherapy in terms of reduction of both ischaemic and bleeding risk in patients who have tolerated 6-18 months of DAPT post-PCI without ischaemic or haemorrhagic complications persists through longer-term follow-up. The initial two-year results had suggested a numerically higher rate of all-cause mortality in the clopidogrel group, however, though the trial was not powered for mortality, this trend is not borne out in the longer-term follow-up, and, therefore, was likely simply a chance finding.
Interestingly, in the post-24-month period, aspirin was discontinued for both gastrointestinal discomfort or minor bleeding and self-discontinued more frequently than clopidogrel. Given the importance of compliance with anti-platelet therapy, this raises the question with regard to clopidogrel being a more acceptable medication to patients in the longer term.
As with any analysis, there is a number of limitations that must be borne in mind, most importantly, the trial is not powered to assess outcomes in subgroups. The study was carried out in exclusively Korean centres, therefore the results may not be generalisable to non-East Asian populations. Clopidogrel non-responsiveness remains a concern due to the risk of stent thrombosis, however, platelet reactivity testing is not routinely recommended. Furthermore, by nature of the trial design patients who had an ischaemic or haemorrhagic event in the 6-18 months post-PCI will not have been enrolled in this study. Therefore this strategy, presently, should only be considered in patients who are event-free at discontinuation of DAPT.
Overall, this trial further supports the benefit of monotherapy with a P2Y12 inhibitor above aspirin in this population post-PCI. Given that the median follow-up was 5.8 years in this trial and antiplatelet therapy is typically prescribed lifelong further follow-up of the population will be of interest to understand the natural history of clopidogrel monotherapy post-PCI. To date, the optimal long-term antiplatelet strategy in all populations remains undetermined and clinicians must carefully assess individual patients' bleeding and ischaemic risks when prescribing and reviewing antiplatelet therapy.
References
- Koo BK, Kang J, Park KW, Rhee TM, Yang HM, Won KB, et al. Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): an investigator-initiated, prospective, randomised, open-label, multicentre trial. Lancet. 2021 May 14;
- Baber U, Dangas G, Angiolillo DJ, Cohen DJ, Sharma SK, Nicolas J, et al. Ticagrelor alone vs. ticagrelor plus aspirin following percutaneous coronary intervention in patients with non-ST-segment elevation acute coronary syndromes: TWILIGHT-ACS. Eur Heart J. 2020 Oct 1;41(37):3533–45.