07 Jun 2022
Biolimus-coated balloon in small-vessel coronary artery disease - The BIO-RISE CHINA study
Selected in JACC: Cardiovascular Interventions by N. Ryan
This trial aimed to assess the safety and efficacy of a biolimus coated balloon in patients with small vessel CAD undergoing PCI.
References
Authors
Kai Xu, GuoSheng Fu, Qian Tong, Bin Liu, XueBin Han, Jun Zhang, GenShan Ma, Qing Yang, Hui Li, Yujie Zhou, Quanmin Jing, Yi Li, and YaLing Han
Reference
J Am Coll Cardiol Intv. May 25, 2022. Epublished DOI: 10.1016/j.jcin.2022.03.024
Published
25 May 2022
Link
Read the abstract
Reviewer
Latest contributions
Balloon lithotripsy added to conventional preparation before stent implantation in severely calcified coronary lesions Impact of pullback pressure gradient on clinical outcomes after percutaneous coronary interventions Validation of intravascular ultrasound-defined optimal stent expansion criteria for favorable 1-year clinical outcomesMy Comment
Why this study - the rationale / objective:
The BIO-RISE CHINA Trial is a prospective, randomised, multicentre, superiority trial comparing the safety and efficacy of a biolimus-coated balloon versus balloon angioplasty alone in patients with small vessel coronary artery disease.
Drug-eluting stents (DES) are currently the gold standard for patients undergoing percutaneous coronary intervention.
In certain subgroups, the concept of ‘leave nothing behind’ is an attractive strategy with drug-coated balloons (DCB) shown to be equivalent to DES in patients with in-stent restenosis. DCB’s have been shown to be non-inferior to 1st generation DES in small vessels1 and to have lower late lumen loss compared to new generation DES in a further trial2.
However, due to the limited data, the use of DCBs in de-novo coronary stenosis is not incorporated into current clinical guidelines.
Furthermore, all RCTs to date have used paclitaxel-coated balloons, though sirolimus-coated balloons are in use in clinical practice.
This trial aimed to assess the safety and efficacy of a biolimus-coated balloon in patients with small-vessel coronary artery disease undergoing PCI.
How was it executed? - the methodology:
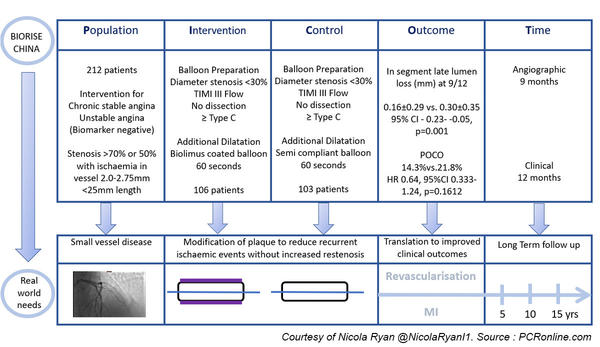
Patients undergoing angiography for chronic stable angina or unstable angina (biomarker negative) with angiographic evidence of atherosclerosis, > 70 % or > 50 % with evidence of ischaemia, in vessels 2.0-2.75 mm in diameter and a length < 25 mm were eligible for inclusion.
After successful preparation; diameter stenosis < 30 %, TIMI III flow, no dissection ≥ type C, patients were randomized 1:1 to additional dilatation with a biolimus-coated balloon or additional dilatation with a semi-compliant balloon.
Patients underwent angiographic follow-up at nine months and clinical follow-up at 30 days, 6, 9, and 12 months.
- The primary endpoint was in-segment late lumen loss at 9 months as assessed by QCA
- Secondary endpoints included binary restenosis, lesion, device and procedural success.
- Patient orientated clinical outcome (POCO) included all-cause death, any MI, and any revascularisation
What is the main result?
Overall, between May 2018 and July 2020, 212 patients were included in the trial, 60 % presenting with unstable angina, 40 % with chronic stable angina, the majority were male in their 60’s with a high prevalence of traditional CVRFs.
Three patients were excluded due to use of the incorrect device, randomisation to biolimus-coated balloon occurred in 106, and semi-compliant balloon in 103.
In both groups, 3 patients underwent stent implantation, angiographic follow-up was available in 89 patients in the biolimus-coated balloon group, and 88 patients in the semi-compliant balloon group.
Clinical follow-up was available in 99.1 % and 98.1 % of patients in the biolimus-coated balloon and semi-compliant balloon groups respectively.
- There was significantly less late lumen loss in the biolimus-coated balloon group. (0.16 mm ± 0.29 mm vs. 0.30 mm ± 0.35 mm, 95 % CI -0.23- -0.05, p = 0.001)
- The biolimus group had more late lumen enlargement than the semi-compliant balloon group (29.7 % vs. 9.8 %, p = 0.007), with lower rates of binary restenosis (0.0 % vs 28.3 %, p = 0.001)
- The rates of TLR were numerically but not statistically lower in the biolimus-coated balloon group (6.7 % vs. 13.9 %, HR 0.47, 95 % CI 0.19-1.16, p = 0.088)
- Similarly POCO did not reach statistical significance but was numerically lower in the biolimus coated balloon group (14.3 % vs. 21.8 %, HR 0.64, 95 % CI 0.333-1.24, p = 0.1612)
PICOT analysis of the BIO-RISE CHINA Study
Critical reading and the relevance for clinical practice:
The results of this study show that biolimus-coated balloon angioplasty is superior to balloon angioplasty alone in terms of late lumen loss in patients with small vessel coronary artery disease.
Of note, the authors compared the biolimus-coated balloon to balloon angioplasty alone rather than a paclitaxel-eluting balloon or a DES, therefore it is impossible to draw comparisons between current guidelines (implantation of DES) or currently available DCB’s, which whilst not guideline-recommended are used in real-world clinical practice3.
Despite the lower rates of late lumen loss and increased late lumen enlargement, other studies have shown that paclitaxel-coated balloons have lower rates of late lumen loss and increased late lumen enlargement than those demonstrated in this trial. Importantly, however, it is not possible to draw direct comparisons between biolimus-coated and paclitaxel-coated balloons without randomised control trials.
The positive remodelling seen with the biolimus-coated balloon is potentially attractive, particularly in the field of CTO PCI, where ballooning a small distal vessel and carrying out a second optimisation procedure may be preferable to stenting a small negatively remodelled vessel. However again, comparison with paclitaxel-coated balloons in head-to-head trials is required to determine if this is a useful hypothesis.
It must be borne in mind that there is a number of limitations to this trial, including the relatively small numbers of patients included, and the fact that the control arm was balloon angioplasty alone limiting the comparison with other technologies.
Further research comparing biolimus-coated and paclitaxel-coated balloons in de-novo coronary stenosis is required to determine the role of biolimus-coated balloons in clinical practice.
Presently, the use of DCB’s in everyday clinical practice needs to be carefully considered and individualised to the patient.
References
- Jeger RV, Farah A, Ohlow MA, Mangner N, Möbius-Winkler S, Leibundgut G, et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): an open-label randomised non-inferiority trial. The Lancet [Internet]. 2018 Aug [cited 2018 Aug 29]; Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673618317197
- Cortese B, Di Palma G, Guimaraes MG, Piraino D, Orrego PS, Buccheri D, et al. Drug-Coated Balloon Versus Drug-Eluting Stent for Small Coronary Vessel Disease: PICCOLETO II Randomized Clinical Trial. JACC: Cardiovascular Interventions. 2020 Dec 28;13(24):2840–9.
- Silverio A, Buccheri S, Venetsanos D, Alfredsson J, Lagerqvist B, Persson J, et al. Percutaneous Treatment and Outcomes of Small Coronary Vessels: A SCAAR Report. JACC: Cardiovascular Interventions. 2020 Apr 13;13(7):793–804.






1 comment
included lesions are relatively small & short, so if they not have any evidence of inducible ischemia, PCI could not give any benefit on outcomes. Some physiologic assessment should be performed for better designed study?