03 Jun 2022
Definitions and standardized endpoints for treatment of coronary bifurcations: Bif-ARC
Selected in EuroIntervention by A. N. Calik
The current consensus document, which is prepared by the collaboration of the most renowned interventional cardiology societies and academic research organizations, aims to provide standardized definitions for bifurcation lesions.
References
Authors
Mattia Lunardi, Yves Louvard,Thierry Lefèvre, Goran Stankovic, Francesco Burzotta, Ghassan S. Kassab, Jens Flensted Lassen, Olivier Darremont, Scot Garg, Bon-Kwon Koo, Niels Ramsing Holm, Thomas W. Johnson, Manuel Pan, Yiannis S. Chatzizisis, Adrian P. Banning, Alaide Chieffo, Dariusz Dudek, David Hildick-Smith, Jérome Garot, Timothy D. Henry, George Dangas, Gregg Stone, Mitchell W. Krucoff, Donald Cutlip, Roxana Mehran, William Wijns, Faisal Sharif, Patrick W. Serruys, Yoshinobu Onuma
Reference
May 18, 2022 | 10.4244/EIJ-E-22-00018
Published
18 May 2022
Link
Read the abstract
Reviewer
My Comment
Definitions and Standardized Endpoints for Treatment of Coronary Bifurcations Expert Consensus Document By The Bifurcation Academic Research Consortium (Bif-ARC)
Introduction
The current consensus document, which is prepared by the collaboration of the most renowned interventional cardiology societies and academic research organizations, aims to provide standardized definitions for bifurcation lesions; the criteria to judge the side branch relevance; the procedural, mechanistic, and clinical endpoints for every type of bifurcation study; and the follow-up methods to facilitate pooled analyses and the effective comparison of data in the future, improving the clinical relevance of trials in bifurcation lesions, and the quality of care in this subset of patients (Figure 1).
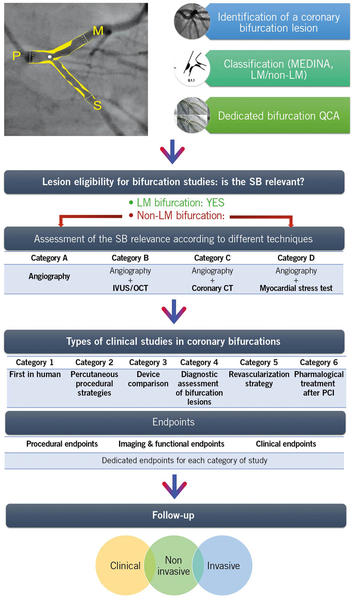
Figure 1. Summary of Bif-ARC Recommendations. After identifying a bifurcation lesion, this should be classified according to MEDINA classification and the left main (LM) involvement. Dedicated bifurcation quantitative coronary analysis (QCA) is strongly advised to be used by sites and core laboratories for quantitative assessment. To evaluate the eligibility of the lesion in a bifurcation trial, its side branch (SB) should be proven to be clinically relevant through different diagnostic techniques according to their availability in the center and the quality and type of the study. The Bifurcation Academic Research Consortium (Bif-ARC) recommends 6 classes of study type, based on the investigation. For every category of study, dedicated endpoints are provided and classified in 3 groups. The follow-up includes clinical, noninvasive, and invasive assessment.
Source: EuroIntervention
Anatomical definitions and classification of coronary bifurcation lesions
Bifurcation Academic Research Consortium (Bif-ARC) acknowledges the coronary bifurcation definitions of the last European Bifurcation Club consensus. Accordingly, a bifurcation lesion is defined as coronary stenosis adjacent to and /or involving an adequate-sized side branch (SB) (≥ 2.0 mm in RefD). The lesion is considered significant when its percentage diameter stenosis (% DS) is > 50, and the minimum lumen diameter (MLD) in at least 1 of the 3 segments is located ≤ 4 mm from the point of bifurcation (POB) (Figure 2).
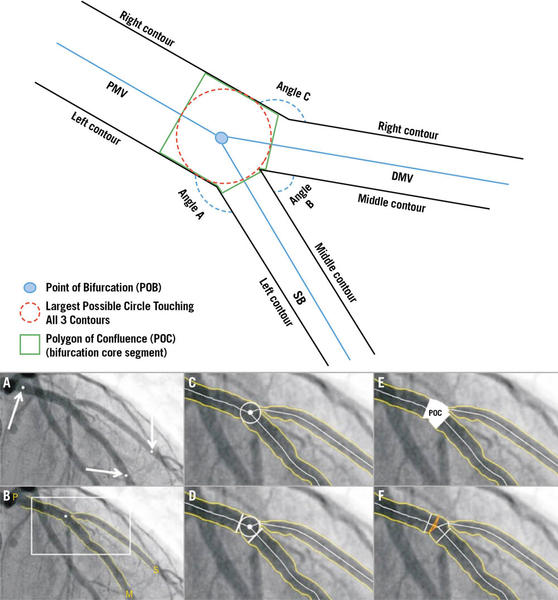
Figure 2. Coronary bifurcation composition. (Top) Schematic representation of coronary bifurcation components. Angle A: access; Angle B: between; Angle C: PM-DM vessel angle. (A to F) Case example of coronary bifurcation on angiography analyzed with dedicated bifurcation quantitative coronary analysis. Arrows: identification of PMV, DMV, and SB by the analyst. DMV: distal main vessel; PMV: proximal main vessel; POB: point of bifurcation; POC: polygon of confluence; SB: side branch.
Source: EuroIntervention
Given its simplicity and prognostic relevance, the consensus document recommends using MEDINA classification. It identifies “true” bifurcation lesions involving a significant (≥ 50 %) diameter stenosis both in the main vessel (MV) and SB (ie, MEDINA 1,1,1; 1,0,1; or 0,1,1), and “non-true” lesions in all other cases. Further, the utilization of dedicated bifurcation quantitative coronary analysis (QCA) software (either onsite or by core laboratory analysis), and intravascular imaging (IVUS & OCT), which have been reported to be more accurate in detecting atheroma compared with invasive angiography, are strongly recommended.
Bif-ARC also classifies the bifurcation lesions in LM and non-LM bifurcations, in addition to SB size and atherosclerotic involvement. SBs with a diameter < 2.75 mm are “minor” SBs, whereas those with a diameter ≥ 2.75 mm are “major” SBs. The length of an SB lesion influences the complexity of SB intervention; as such, an SB lesion ≥ 10 mm renders the treatment potentially more challenging.
Target bifurcation lesions for bifurcation studies
The SB's relevance is fundamental, not only to defining the bifurcation lesion but also for inclusion in a dedicated bifurcation study. As demonstrated in previous studies, a 10 % cutoff in ischemic myocardium represents a critical benchmark combining the ischemic and prognostic implications of a coronary lesion. Thus, the consensus document proposes that an SB should be defined as "relevant" if symptoms stem from a large amount (>10%) of ischemic SB-related myocardium, impacting prognosis.
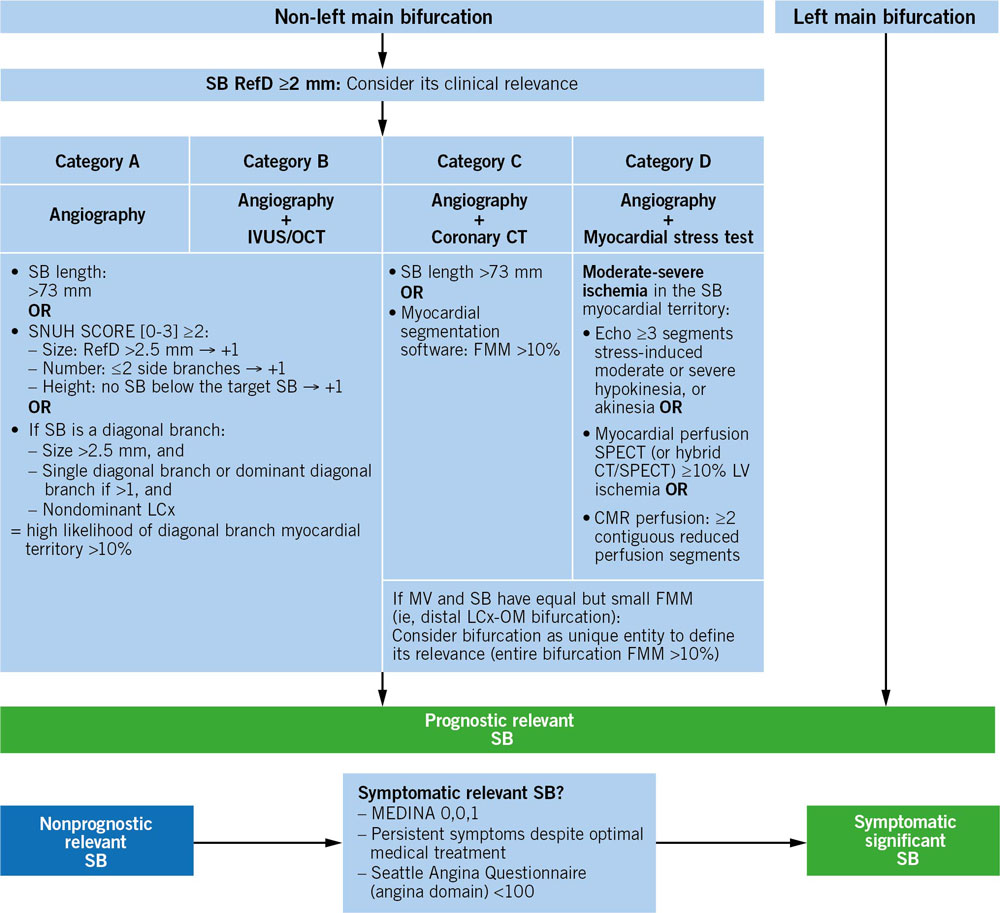
Bif-ARC proposes a standardized algorithm based on available diagnostic techniques (Angiography, IVUS/OCT, coronary CT, myocardial stress test) to classify bifurcation studies into different categories, which will facilitate comparisons across studies of similar technical requirements and help estimate the SB-related myocardium at risk of non-LM bifurcations (the SBs of LM bifurcations are always considered prognostically relevant) (Figure 3).
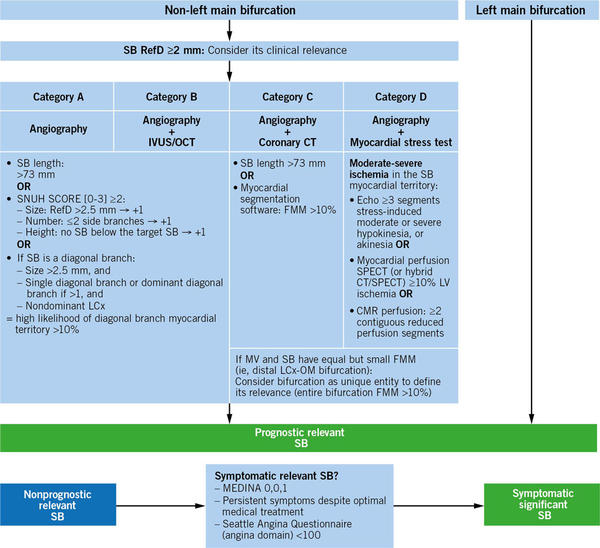
Figure 3. Algorithm to determine the lesion eligibility according to the sb relevance. CMR: cardiac magnetic resonance; CT: computed tomography; FMM: fractional myocardial mass; IVUS: intravascular ultrasound; LCx: left circumflex coronary artery; MV: main vessel; OCT: optical coherence tomography; OM: obtuse marginal branch; RefD: reference diameter; SB: side branch; SPECT: single-photon emission computed tomography
Source: EuroIntervention
For diagonal SBs, using the SNuH (size, number, highest) score, a simple anatomical scoring system based on angiography to estimate the mass of myocardium at risk, is recommended.
Bif-ARC aims to ease comparability between studies addressing complex lesions and defines complexity according to the evaluation method (angiography, intravascular imaging and coronary CT). Briefly, having a true bifurcation lesion (MEDINA 1,1,1; 1,0,1; 0,1,1) and one of the following criterion identifies a complex bifurcation lesion:
- SB disease length ≥10 mm
- Calcified lesion
- Thrombotic lesion
- Difficult SB access (higher risk if bifurcation angle A < 90°)
- Calcium arc > 60° at the culprit lesion site (for IVUS/OCT and coronary CT)
Invasive Functional Assessment & Intravascular Imaging of Bifurcation Lesions
Invasive functional assessment of the bifurcation lesions may be clinically required to assess ischemia or mandated by the study protocol. The consensus document recommends using invasive functional assessment as follows:
- Before intervention: to evaluate the functional significance of an MV stenosis or pure SB stenosis (MEDINA 0,0,1) when ischemia has not been confirmed elsewhere;
- During intervention: to decide whether additional interventions are required in a jailed SB (ie, the occurrence of tight ostial stenosis of SB after crossover stenting of the MV;
- After intervention: to assess the functional significance of a jailed SB or assess procedural success in the MV and the SB, if treated. A jailed pressure wire was shown utilizable for the assessment in the first case.
Beyond offering a better definition of plaque composition (eg, localizing and quantifying calcium and lipid), intracoronary imaging provides crucial periprocedural information (eg, lesion coverage, wire positions, stent expansion, and strut apposition) to help optimize treatment. Therefore, Bif-ARC supports the use of intravascular imaging as an adjunctive technique in bifurcation trials.
Types of clinical studies in coronary bifurcations and endpoint definitions
As a primary classification, Bif-ARC suggests separate trials of LM and non-LM bifurcations in order to prevent including both types of bifurcation lesions in the same study. In particular cases where the investigators desire to include both types, their inclusion in the study should be stratified according to that variable, or a stratified randomization should be considered.
Beyond this, Bif-ARC proposes the following classification of studies:
- First-in-human studies: any study introducing a new device for use in humans for the first time (ie, specifically designed and dedicated to treating bifurcation lesions).
- Comparison of percutaneous procedural strategies: studies comparing different percutaneous techniques to treat a bifurcation lesion belong to this category (eg, DK-Crush [Double Kissing Crush versus Provisional Stenting for Left Main Distal Bifurcation Lesions] and EBC MAIN [The European Bifurcation Club Left Main Study] trials).
- Device comparisons: these studies investigate new or existing devices (both stents and balloons, either dedicated bifurcation or not).
- Diagnostic assessment of bifurcation lesions: this category includes clinical trials aiming to compare different diagnostic techniques (both imaging and functional; ie, 2D angiography vs 3 dimensional [3D] coronary CT angiography or invasive vs image-based physiological assessment).
- Revascularization strategy: these studies will compare percutaneous vs surgical revascularization treatments and is especially important for LM bifurcation lesions (eg, LM bifurcation subgroup of the SYNTAX [Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery] trial, EXCEL [Evaluation of XIENCE versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization] trial).
- Pharmacological treatment after percutaneous coronary intervention: studies comparing different medical strategies after bifurcation PCI (single vs dual antiplatelet therapy [DAPT], short vs long DAPT, DAPT vs triple therapy, different targets of platelet inhibition, etc) will be included in this category.
The document also underscores the importance of the bench test studies and recommends that any novel technique or device should first be tested in bench studies before undertaking a clinical study.
For every study category (1 to 6), Bif-ARC proposes 3 endpoints:
- Procedural: procedure-related outcomes, endpoints regarding the complexity of the procedure (eg, procedural time, x-ray exposure, etc), and health-economic data.
- Imaging and functional: mechanistic endpoints based on angiographic, intravascular, noninvasive imaging, or functional evaluation. These endpoints serve as reports of common angiographic complications, immediate imaging, and function-based results and are preferably assessed by the core lab.
- Clinical endpoints: encompass efficacy and safety endpoints, and depending on the study, patient or device-related endpoints should be included.
In trials comparing procedural strategies, devices, and diagnostic assessment studies (categories 2, 3, and 4) procedural, imaging, and functional endpoints have particular relevance and should be defined as primary endpoints. In trials focusing on pharmacological regimens or comparing different types of revascularization strategies (categories 5 and 6) clinical composites should be set as primary endpoints, including safety (eg, bleeding events) and ischemic endpoints.
Individual Endpoints
- Device and Procedural Success: device success is defined as the composite of successful delivery of the first assigned device at the intended target bifurcation, successful withdrawal of its delivery system, and a final in-stent/scaffold % DS < 20 in each stented segment of the bifurcation by visual assessment or < 30 % by bifurcation QCA61 (< 50 % in case of balloon angioplasty alone). When the use of intravascular imaging is mandated by the study protocol, device success is defined by a final minimum stent area > 80 % of the reference vessel area in each stented segment of the bifurcation.
- Periprocedural myocardial infarction (PMI): PMI is defined by either an absolute rise ≥ 35 upper limit of normal (ULN) threshold for type T hs-cTn plus clinical evidence of MI or an absolute cTn rise ≥ 70 ULN as a stand-alone criterion within 48 hours of the PCI or coronary artery bypass graft (CABG).
- Bleeding: Bif-ARC supports using the ARC-HBR (Academic Research Consortium High Bleeding Risk) tool to evaluate patients’ bleeding risk and their ischemia/bleeding tradeoff, although further validation in the specific context such as bifurcation PCI is needed.
- Target bifurcation revascularization (TBR): the target bifurcation is commonly considered as the coronary bifurcation segment treated during the index procedure plus 5 mm from the stent edges or the site of the balloon angioplasty, applied to both the MV and SB. A target bifurcation revascularization (TBR) is defined as a repeat revascularization of the target bifurcation by PCI or bypass surgery of the target vessel(s), performed for restenosis or another complication of the target bifurcation.
- Definite stent thrombosis: angiographic confirmation of the presence of a thrombus that originates in the stent or in the segment 5 mm proximal or distal to the stent or in the side branch originating from the stented segment and the presence of at least 1 of the following criteria:
- Acute onset of ischemic symptoms at rest
- New electrocardiographic changes suggestive of acute ischemia
- Typical rise and fall in cardiac biomarkers (refer to the definition of spontaneous myocardial infarction)
- OR Pathological confirmation:
- Evidence of recent thrombus within the stent determined at autopsy
- Examination of tissue retrieved following thrombectomy (visual/histology)
Follow-up Methods
Bif-ARC recommends carrying out follow-up on a 3-level basis:
- Clinical and patient level (eg, clinical and patient-reported endpoints); Minimum of 12-month clinical follow-up is recommended when no angiographic follow-up is required. Also, the patient-related outcomes, including quality of life (QoL) assessment, are recommended. In studies comparing surgical vs percutaneous revascularizations, Bif-ARC recommends extending clinical follow-up to 10 years.
- Noninvasive testing (eg, ischemia tests, coronary CT); Noninvasive testing should be undertaken whenever a clinical suspicion of recurrent ischemia exists or if mandated by the study protocol.
- Invasive testing (eg, coronary angiography); In cases of invasive follow-up, core lab analysis is recommended. When required by the study protocol or for clinical reasons, IVUS or OCT can be used. Functional assessment of the target bifurcation is advised to measure its “functional deterioration,” defined as a reduction in the functional values compared with the postprocedural values.
Impact on daily practice
In conclusion, this document aims to provide standardized definitions and criteria for use in studies of coronary bifurcation lesions, from diagnosis through follow-up, which will improve their relevance and quality of care for patients with coronary bifurcation disease.
Questions to the authors
Mattia Lunardi, Patrick W. Serruys, and Yoshinobu Onuma agreed to answer Ali Nazmi Calik's questions to go even further! Discover here their answers without taboos.
Considering the importance of the operators’ experience in treating bifurcation lesions, should such a criterion be set while conducting coronary bifurcation trials?
The operators’ experience in performing bifurcation PCI is a major factor influencing the outcomes. It has been shown that patients who underwent left main PCI by high-volume and experienced operators had better short- and long-term prognoses.
The consensus, indeed, recommends reporting the volume of LM bifurcation PCI/year of the center, when performing LM bifurcation studies. Expanding the collection of such data to all bifurcation types is, however, feasible and might offer important future insights. Performing surveys to understand the bifurcation PCI volume before centers selection for a trial might also help to improve the quality of bifurcation studies.
Considering the reimbursement issues, particularly in the middle to low-income countries, how can the usage of intravascular imaging guidance be improved to achieve better results?
Given the economic concerns of low-income countries, a wise use of any optional (but recommended) tool is valuable.
The first advice is to limit the use of intravascular imaging for complex bifurcations, e.g. where angiography is limited to depict the anatomy, or in cases where plaque distribution and type (e.g. calcified lesions) may change the procedural strategy, or yet when optimisation is important (like left main bifurcation).
Once on the table of the operator, IVUS or OCT catheters should be used to guide each procedural step: diagnosis, PCI planning, and PCI optimization.
Hence, the decision to use intravascular imaging should be taken before starting the PCI, in order to get all the benefits from the imaging catheter, including indeed the diagnostic part.
Of note, the choice of either IVUS or OCT is based on both center availability, operators’ experience, and the Bif-ARC recommendations according to the lesion anatomy.
In all cases not supported by imaging, the use of fractal formulas applied to angiography-derived measurements (e.g. Finet’s Law) helps to get more accurate reference diameters.
As for the physiological assessment of the SB ostial lesions after crossover stenting of the main vessel, are there any other options than the jailed wire technique? Can dual-lumen microcatheters be used after rewiring with a softer tip guidewire to exchange with the FFR guidewires?
Definitely!
The jailed pressure wire technique mentioned in the consensus is an option, that while has been shown feasible in a small series of patients, it might raise safety concerns among some operators regarding potential damages to the wire, leading to procedural complications.
Alternatively, the operator should firstly attempt the rewiring of the SB with a pressure wire after the POT, that however may be challenging given the mechanical properties of this type of wire.
If the rewiring with the pressure wire is not possible, the use of a dual-lumen (or even normal) microcatheter can help to exchange a softer wire with the pressure wire.
In this case, the operator should carefully perform the normalization of the pressure wire placing the sensor before the SB ostium, without losing the position.







2 comments
Great summary! Thanks Nazmi
Thank you, Erik.