21 Apr 2022
Prophylactic Rivaroxaban therapy for left ventricular thrombus after anterior ST segment elevation myocardial infarction
Selected in JACC: Cardiovascular Interventions by E. Asher
The aim of the current study was to investigate the effects of rivaroxaban on left ventricle thromboprophylaxis in patients with anterior STEMI.
References
Authors
Zhongfan Zhang , Daoyuan Si , Qian Zhang , Lina Jin , Haikuo Zheng , Ming Qu , Miao Yu , Zhenya Jiang , Delin Li , Souping Li , Ping Yang , Yuquan He , and Wenqi Zhang
Reference
J Am Coll Cardiol Intv. 2022 Apr, 15 (8) 861–872
Published
15 April 2022
Link
Read the abstract
Reviewer
My Comment
Why this study – the rationale/objective?
Left ventricular thrombus (LVT) usually appears within 1 month after ST-segment elevation myocardial infarction (STEMI) and mostly after anterior STEMI.
LVT incidence in patients with anterior STEMI is roughly 4 % to 26 %. It complicates acute myocardial infarction and is associated with a higher incidence of poor outcomes.
Conventional triple anticoagulation (combination vitamin K antagonists [VKAs] and dual antiplatelet therapy [DAPT]) is recommended to prevent LVT in patients at high risk of STEMI, although it is not supported by high-quality evidence.
The aim of the current study was to investigate the effects of rivaroxaban on left ventricle thromboprophylaxis in patients with anterior STEMI.
How was it executed? - the methodology
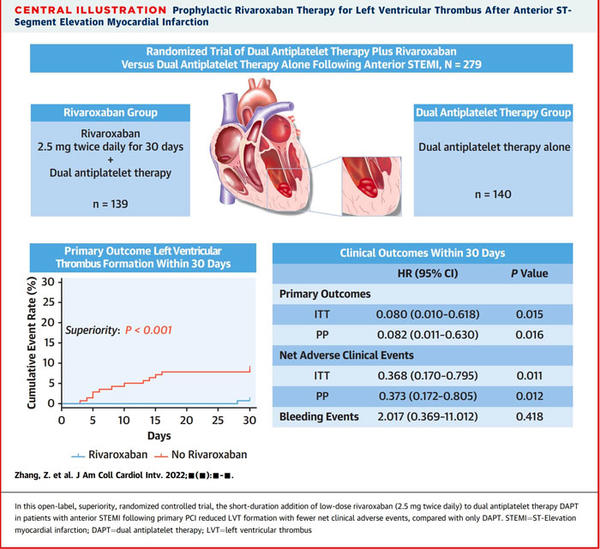
The study was an investigator-initiated, single-center, open-label, superiority, randomized controlled trial that randomly assigned 279 patients with anterior STEMI who had undergone primary percutaneous coronary intervention to receive, in a 1:1 ratio, low-dose rivaroxaban (2.5 mg twice daily for 30 days) and DAPT or DAPT only. All patients were followed up for 90 to 180 days to assess the short-term prognosis with short-duration use (30 days) of rivaroxaban.
Exclusion criteria included:
- intracranial, gastrointestinal, or urogenital bleeding within 3 months;
- a drug allergy;
- severe liver and kidney diseases, chronic renal failure, serum creatinine > 3.0 mg/dL, or transaminase exceeded the reference value by more than 3 times;
- currently taking rivaroxaban or requiring long-term anticoagulation therapy;
- pregnancy and malignant tumors;
- confirmed or suspected hematology diseases (except mild or moderate anemia);
- thrombocytopenia (platelets < 100,000 / ml);
- a high risk of bleeding;
- a serious cardiac condition (i.e., ventricular arrhythmias refractory before randomization or cardiac shock);
- and older than 75 years or less than 18 years.
LVT Diagnosis:
LVT was routinely evaluated by echocardiography and the results were evaluated by 2 cardiologists.
If the diagnosis was suspicious, the LVT was further assessed with cardiac magnetic resonance or contrast echocardiography.
Primary outcome:
LVT formation within 30 days.
Secondary outcomes:
Composite of net adverse clinical events (NACEs) within 30 days consisting of all-cause mortality, LVT, systemic embolism (SE), rehospitalization for cardiovascular events, or bleeding.
Other secondary outcomes included NACEs and LVT formation throughout the follow-up period.
Safety outcome:
A composite of major, clinically relevant nonmajor, and minor bleeding within 30 days, which was defined by the International Society on Thrombosis and Hemostasis.
What is the main result?
- Overall, 279 patients underwent randomization; 139 patients were assigned to rivaroxaban and DAPT therapy, and 140 were assigned to only DAPT therapy. In total, 32 patients were lost to follow-up after the admission visit in all randomized patients, 14 withdrew consent during the intervention, and 5 discontinued the drug on their own.
- The addition of low-dose rivaroxaban to DAPT reduced LVT formation within 30 days compared with only DAPT (0.7 % vs 8.6 %; HR: 0.08; 95 % CI: 0.01-0.62; P < 0.015; P < 0.001 for superiority).
- Net clinical adverse events were lower within 30 days in the rivaroxaban group versus those in the only DAPT group (6.5 % and 16.4 %, respectively; HR:0.37; 95 % CI: 0.17-0.80; P < 0.011). and remained relatively low throughout the follow-up period.
- SE occurred less frequently in patients in the rivaroxaban group than in those in the DAPT-only group but with no significant differences (0.7 % and 2.9 %, respectively; HR: 0.51; 95 % CI: 0.09-2.69; P = 0.415).
- There were no significant differences in bleeding events between the 2 groups in 30 days and 180 days. However, 1 case of intracranial hemorrhage (major bleeding) occurred in the rivaroxaban group within 30 days.
- All-cause mortality, SE, rehospitalization for cardiovascular events, and bleeding showed no significant differences throughout the follow-up period.
Prophylactic Rivaroxaban therapy for left ventricular thrombus after anterior ST segment elevation myocardial infarction
Source: JACC - Cardiovascular Interventions
Critical reading and the relevance for clinical practice?
The study found that among patients with anterior STEMI following primary PCI, the addition of low-dose rivaroxaban (2.5 mg twice daily for 30 days) to DAPT reduced LVT formation within 30 days compared with only DAPT (1 patient vs 12 patients).
Even after discontinuing rivaroxaban, relatively fewer NACEs were observed in the rivaroxaban group during the follow-up period. Moreover, the current recognition of the prognostic relevance of major bleeding from the triple therapy with VKAs questions the suggestion of prophylactic anticoagulation in patients with wall motion abnormalities following an acute anterior STEMI. Hence, Given the positive results of rivaroxaban in various clinical settings, the authors think it is reasonable to extrapolate its efficacy to left ventricle thromboprophylaxis.
It is noteworthy that the rivaroxaban group also observed a relatively lower incidence of SE than the DAPT-only group but was not statistically significant, which may be related to the small number of events in the study.
Should common practice and guidelines be changed?
The study has several limitations:
First, considering the low incidence of LVT after STEMI in modern time, the authors selected patients with anterior STEMI.
Second, a relatively high dropout rate was presented in the trial because of the impact of the coronavirus disease pandemic.
Third, the study designed a 30-day duration for prophylactic anticoagulation. Based on the NACE results (most occurred within 90 days) observed in this trial, perhaps the appropriate extension of anticoagulation duration will be more beneficial under close follow-up.
Fourth, with a cohort size of only 279 patients, this study was underpowered to detect the relative differences in the individual components of NACEs.
The authors concluded that the results support the short-duration addition of low-dose rivaroxaban (2.5 mg twice daily) to DAPT to prevent LVT formation in patients with anterior STEMI who had undergone coronary interventions.
-> What is your approach regarding the use of prophylactic low-dose rivaroxaban to prevent LVT complicating anterior STEMI? Would you start using it as a routine treatment for such patients?




2 comments
Concept is good. But study patient numbers are small with high drop out. Won’t change our practice. Needs a larger study before any guidelines. Worth studying with other NOACs such as Apixaban to reduce bleeding risk
Have had 3 patients with Ant. STEMI and LVT. The first had AF as well. I did stenting and initiated Triple anticoagulants; Clopidogrel 75+ASA 81+ Rivaroxaban 15 mgs. Follow-up echo showed resolution of LVT after 1 month. The other two patients, were similar albeit NO AF. I was encouraged to put a lower Riva at 10 mgs. One LVT disappeared after almost 3 months, the other LVT stayed in place with near organisation to the apex. I maintained the Riva till date for both patients; the apex is akinetic. I also discontinued the ASA and maintained the Clopidogrel