31 Jan 2022
Ticagrelor monotherapy after PCI in high-risk patients with prior MI: a prespecified TWILIGHT substudy
Selected in JACC: Cardiovascular Interventions by N. Ryan
In this prespecified subgroup analysis of the TWILIGHT trial, the authors report the impact of prior MI on treatment effects of ticagrelor monotherapy compared to ticagrelor and aspirin in patients at least three months post PCI...
References
Authors
Mauro Chiarito, Usman Baber, Davide Cao, Samin K. Sharma, George Dangas, Dominick J. Angiolillo, Carlo Briguori, David J. Cohen, Dariusz Dudek, Vladimír Džavík, Javier Escaned, Robert Gil, Christian W. Hamm, Timothy Henry, Kurt Huber, Adnan Kastrati, Upendra Kaul, Ran Kornowski, Mitchell Krucoff, Vijay Kunadian, Shamir R. Mehta, David Moliterno, E. Magnus Ohman, Keith Oldroyd, Gennaro Sardella, Zhang Zhongjie, Samantha Sartori, Giulio Stefanini, Richard Shlofmitz, P. Gabriel Steg, Giora Weisz, Bernhard Witzenbichler, Ya-ling Han, Stuart Pocock, C. Michael Gibson, and Roxana Mehran
Reference
JACC Cardiovasc Interv. 2022 Jan 5;S1936-8798(21)02022-7
Published
January 2022
Link
Read the abstract
Reviewer
Latest contributions
Balloon lithotripsy added to conventional preparation before stent implantation in severely calcified coronary lesions Impact of pullback pressure gradient on clinical outcomes after percutaneous coronary interventions Validation of intravascular ultrasound-defined optimal stent expansion criteria for favorable 1-year clinical outcomesMy Comment
In this prespecified subgroup analysis of the TWILIGHT trial, the authors report the impact of prior MI on treatment effects of ticagrelor monotherapy compared to ticagrelor and aspirin in patients at least three months post PCI with no adverse events and at least one clinical and one angiographic high-risk feature.
Why this study – the rationale/objective?
Dual antiplatelet therapy aims to reduce ischaemic risk after ACS and PCI but comes with a concomitant increased risk of bleeding.
Despite numerous trials, the optimum duration of DAPT post PCI or ACS remains controversial, with the balance of ischaemic versus bleeding risks a key consideration in determining individual patient DAPT duration.
The TWILIGHT study showed that ticagrelor alone, following three months of DAPT without adverse events, reduced bleeding risks in high-risk patients undergoing PCI with no increase in ischaemic events compared to DAPT with ticagrelor and aspirin. Patients with prior MI are known to have an increased ischaemic risk with prior trials showing that long-term DAPT with aspirin and ticagrelor reduces ischaemic risk with a consequent increased bleeding risk.
This sub-study aimed to assess if patients with prior MI benefited from ticagrelor monotherapy in terms of reduced bleeding risk without increased ischaemic risk.
How was it executed? - the methodology
In the TWILIGHT trial, patients with successful PCI with at least one clinical and one angiographic feature associated with high ischaemic or bleeding risk were included.
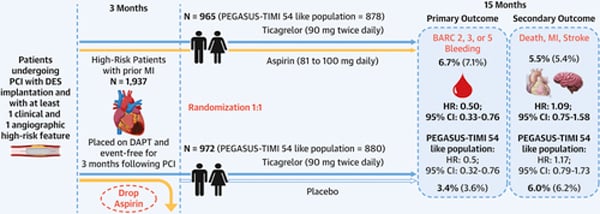
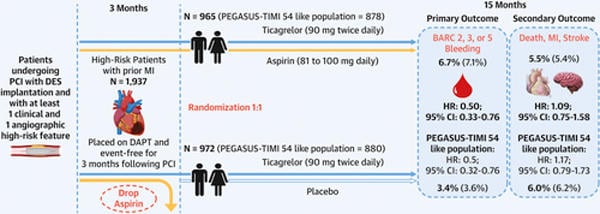
At three months, patients with no major ischaemic or bleeding events were randomised 1:1 to ticagrelor and placebo or aspirin and ticagrelor (7,119 patients). This analysis included 6,532 patients, 1,937 with prior MI (972 received ticagrelor and placebo, 965 received ticagrelor and aspirin), and 4,595 patients without prior MI (2,293 received ticagrelor and placebo, 2,302 received ticagrelor and aspirin).
- The primary endpoint was the first occurrence of a clinically relevant bleeding event; BARC type 2, 3, or 5 bleeding.
- The key secondary endpoint was the first occurrence of death from any cause, nonfatal MI or nonfatal stroke.
- Secondary bleeding endpoints included BARC major bleeding (type 3 or 5), TIMI major or minor bleeding, GUSTO moderate, severe, or life-threatening bleeding, or major bleeding as defined by the International Society on Thrombosis and Haemostasis.
- Other secondary endpoints included the single components of the primary and key secondary endpoints; the composite of cardiovascular death, nonfatal MI, and nonfatal stroke; net adverse clinical events (a composite of BARC type 2, 3, or 5 bleeding; death from any cause; nonfatal MI; and nonfatal stroke); cardiovascular death; and definite or probable stent thrombosis.
Design of the TWILIGHT substudy
Source: JACC - Cardiovascular Interventions
What is the main result?
Overall, 6,532 patients who had undergone coronary stenting, 1,937 with prior MI, and 4,595 without prior MI, were included in this analysis. Patients with prior MI were younger and more commonly male than those without prior MI. Traditional cardiovascular risk factors as well as prior PCI and CABG was more common in the prior MI group.
At one year, the primary endpoint was not significantly different between those with and without prior MI (5.0 % vs 5.5 %, p = 0.68).
- In the prior MI group, the primary endpoint occurred in 3.4 % of patients receiving ticagrelor and placebo, vs. 6.7 % of patients receiving ticagrelor and aspirin (HR 0.50, 95 % CI 0.33-0.76)
- In the group without prior MI, the primary endpoint occurred in 4.2 % of patients receiving ticagrelor and placebo, vs. 7.0 % of patients receiving ticagrelor and aspirin (HR 0.58, 95 % CI 0.45-0.76)
Death, MI, or stroke was higher in the prior MI group than the group without prior MI (5.7 % vs. 3.2 %, p < 0.001), mainly driven by higher non-fatal MI (4.4 % vs. 2.1 %, p < 0.001) - In the prior MI group, there were no differences in the key secondary endpoint between those receiving ticagrelor and placebo, vs. ticagrelor and aspirin (6.0 % vs 5.5 %, HR 1.09, 95 % 0.75-1.58)
- In the group without prior MI, there were no differences in the key secondary endpoint between those receiving ticagrelor and placebo, vs. ticagrelor and aspirin (3.1 % vs 3.3 %, HR 0.92, 95 % 0.67-1.28)
A history of MI did not significantly impact the reduced risk of NACE with ticagrelor monotherapy [(prior MI: 6.8 % vs. 9.9 %, HR 0.67, 95 % CI 0.55-0.83, no prior MI: 8.9 % vs. 11.3 % HR 0.78, 95 % CI 0.59-1.04) p interaction = 0.416].
Critical reading and the relevance for clinical practice
Patients with prior MI have an increased risk of ischaemic events in the longer term, therefore, there has been significant interest in prolonged DAPT to reduce the risk of recurrent ischaemic events. Prior meta-analysis1, as well as PEGASUS-TIMI 542, have shown a reduction in ischaemic events with prolonged DAPT in patients with prior MI, however, this has come at the expense of increased bleeding. Historically, the focus within the PCI community has been on the ischaemic risk post PCI however, it is now understood that bleeding carries important morbidity and mortality, therefore, minimising bleeding without increasing ischaemic risk is a careful balance.
The TWILIGHT trial included patients with high bleeding or ischaemic risk factors. The results of this subgroup analysis show that patients with a history of prior MI were at increased risk of ischaemic events with a similar risk of bleeding events compared to those without prior MI. Ticagrelor monotherapy, compared to DAPT with aspirin and ticagrelor, reduced bleeding events with no concomitant increased ischaemic risk in both patients with and without prior MI.
An important caveat to this conclusion is that the TWILIGHT study was not powered to detect differences in ischaemic events rather it was a prespecified noninferiority hypothesis, therefore, the ischaemic risk can only be considered hypothesis generation and requires further adequately powered trials to support this. Secondly, by nature of the trial design, this subgroup analysis had a relatively small sample size, meaning that it was not possible to draw conclusions with regard to rare but clinically important differences in some adverse events.
The current guidelines recommend discontinuation of dual antiplatelet therapy in most patients at one year with consideration to early discontinuation in patients at high bleeding risk and prolongation in patients with prolongation of DAPT in patients at high ischaemic but low bleeding risk. PEGASUS-TIMI 54 has previously shown that in patients more than one-year post-MI aspirin and low dose ticagrelor (60mg BD) reduced the risk of cardiovascular death, MI, or stroke, with an increased risk of major bleeding. Assessing only those who met the PEGASUS-TIMI 54 inclusion criteria within this analysis ticagrelor monotherapy reduced bleeding risk (HR 0.5, 95 % CI 0.32-0.76) with no increased ischaemic risk (HR 1.17, 95 % CI 0.79-1.73).
This sub-analysis provides important hypothesis-generating data suggesting that ticagrelor monotherapy following three months of DAPT without significant events may be beneficial in selected patients with prior MI undergoing PCI.
Importantly, the benefit of ticagrelor monotherapy cannot be extrapolated to suggest monotherapy with any P2Y12 inhibitor post PCI will produce the same result. Furthermore, a number of patients are non-responders to clopidogrel, therefore, monotherapy may be harmful in their case. Further research is required to understand the risks and benefits of ticagrelor monotherapy and indeed P2Y12 inhibitor monotherapy in patients with prior MI in the longer term.
Current data suggests abbreviated DAPT in HBR populations is a safe and efficacious strategy, this study suggests that short DAPT followed by ticagrelor monotherapy in patients with prior MI may be a safe and efficacious strategy in balancing ischaemic and bleeding risks. However, the approach to both duration of DAPT as well as the monotherapy agent of choice requires further research, in the interim clinicians must carefully assess individual patients bleeding and ischaemic risks when prescribing and reviewing antiplatelet therapy.
References
- Udell JA, Koh M, Qiu F, Austin PC, Wijeysundera HC, Bagai A, et al. Outcomes of Women and Men With Acute Coronary Syndrome Treated With and Without Percutaneous Coronary Revascularization. JAHA [Internet]. 2017 Jan 11 [cited 2021 May 30];6(1). Available from: https://www.ahajournals.org/doi/10.1161/JAHA.116.004319
- Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, et al. Long-Term Use of Ticagrelor in Patients with Prior Myocardial Infarction. N Engl J Med. 2015 May 7;372(19):1791–800.