Ticagrelor with and without aspirin in patients with a prior coronary artery bypass graft undergoing percutaneous coronary intervention: the TWILIGHT-CABG study
Selected in EuroIntervention Journal by N. Ryan
In this post-hoc analysis of the TWILIGHT trial, the authors report the effects of ticagrelor monotherapy compared to ticagrelor and aspirin in patients with prior CABG at least three months post PCI with no adverse events and at least one clinical and one angiographic high-risk feature for ischaemia or bleeding.
References
Authors
Gennaro Sardella, Frans J. Beerkens, George Dangas, Davide Cao, Usman Baber, Samantha Sartori, David J. Cohen, Carlo Briguori, Robert Gil, Johny Nicolas, Zhongjie Zhang, Dariusz Dudek, Vijay Kunadian, Ran Kornowski, Giora Weisz, Bimmer Claessen, Steven O. Marx, Javier Escaned, Kurt Huber, Timothy Collier, David J. Moliterno, E. Magnus Ohman, Mitchell W. Krucoff, Adnan Kastrati, Philippe Gabriel Steg, Dominick J. Angiolillo, Shamir R. Mehta, Richard Shlofmitz, Samin Sharma, Stuart Pocock, Charles Michael Gibson, Roxana Mehran
Reference
DOI: 10.4244/EIJ-D-22-00319
Published
18 August 2022
Link
Read the abstract
Reviewer
Latest contributions
Balloon lithotripsy added to conventional preparation before stent implantation in severely calcified coronary lesions Impact of pullback pressure gradient on clinical outcomes after percutaneous coronary interventions Validation of intravascular ultrasound-defined optimal stent expansion criteria for favorable 1-year clinical outcomesMy Comment
Courtesy of Nicola Ryan @NicolaRyan1 Source: PCRonline.com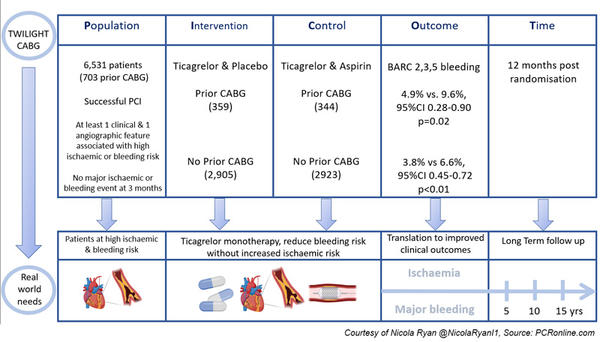
Why this study – the rationale/objective?
Dual antiplatelet therapy aims to reduce ischaemic risk after ACS and PCI, but comes with a concomitant increased risk of bleeding. The Twilight study showed that ticagrelor alone, after three months of DAPT without adverse events, reduced bleeding risks in high-risk patients undergoing PCI, with no increase in ischaemic events compared to DAPT with ticagrelor and aspirin.
Patients with prior CABG have been shown to accelerated native CAD as well as graft failure1. Patient co-morbidities, as well as anatomic complexity, mean that re-do CABG is rare, and patients are commonly treated with PCI if repeat revascularisation is required.
However, PCI post-CABG is associated with worse outcomes than PCI in patients without prior CABG2-4, therefore defining the optimal DAPT strategy is of interest.
This post-hoc analysis aimed to assess if patients with prior CABG benefited from ticagrelor monotherapy in terms of reduced bleeding risk without increased ischaemic risk.
How was it executed? - the methodology
In the Twilight trial, patients with successful PCI with at least one clinical and one angiographic feature associated with high ischaemic or bleeding risk were included.
At three months, patients with no major ischaemic or bleeding events were randomised 1:1 to monotherapy with ticagrelor, or aspirin and ticagrelor (7,119 patients).
This analysis included 6,531 patients 703 with prior CABG (359 received ticagrelor and placebo, 344 received ticagrelor and aspirin) and 5,828 patients without prior CABG (2,905 received ticagrelor and placebo, 2,923 received ticagrelor and aspirin).
- The primary endpoint was the first occurrence of BARC type 2,3 or 5 bleeding between randomisation and 12 months after randomisation.
- The key secondary ischaemic endpoint was a composite of death from any cause, MI or stroke.
- Secondary bleeding endpoints included BARC type. 3 or 5 bleeding, GUSTO moderate, severe or life-threatening bleeding or major bleeding as defined by the ISTH.
What is the main result?
Compared to the population without prior CABG, the CABG group had more cardiovascular risk factors, were older, more commonly male, and more frequently underwent PCI for stable CAD.
- In patients with prior CABG, the primary endpoint occurred less often in patients treated with ticagrelor and placebo compared to ticagrelor and aspirin (4.9 % vs. 9.6 %, HR 0.50, 95 % CI 0.28-0.90, p = 0.02). This was also seen in patients without prior CABG (3.8 % vs 6.6 %, 95 % CI 0.45-0.72, p < 0.01).
- Overall, the key secondary ischaemic endpoint, a composite of death from any cause, MI, or stroke occurred more frequently in patients with prior CABG than those without. (9.4 % vs 3.3 %, adj. HR 2.2, 95 % CI 1.63-2.98, p < 0.001).
- There were no differences in the key secondary ischaemic endpoint in patients with prior CABG treated with ticagrelor and placebo compared to ticagrelor and aspirin (10.0 % vs. 8.7 %, HR 1.14, 95 % CI 0.7-1.87, p = 0.55). Similar results were seen in patients without prior CABG (3.2 % vs. 3.4 %, HR 0.93, 95 % CI 0.70-1.24, p = 0.618).
- Major bleeding (BARC 3 or 5) was reduced in patients with prior CABG treated with ticagrelor and placebo compared to ticagrelor and aspirin (1.1 % vs. 4.5 %, HR 0.25, 95 % CI 0.08-0.76, p = 0.015). Similar reductions were seen in the group without prior CABG (0.9 % vs 1.7 %, HR 0.54, 95 % CI 0.34-0.88, p = 0.012).
Critical reading and the relevance for clinical practice
Patients with prior CABG have an increased risk of adverse outcomes in the longer term post PCI compared to those without, however data supporting the optimal treatment strategy in this population is scarce. Typically, patients undergoing PCI post CABG are older and have more co-morbidities than those undergoing PCI without prior CABG, with a resultant increase in both ischaemic and bleeding risk factors.
There are several important points to be taken from this post-hoc analysis. Firstly, patients with prior CABG have significantly increased ischaemic events post PCI compared to those without. Importantly, there were no differences in ischaemic events in patients treated with ticagrelor and placebo versus ticagrelor and aspirin. Secondly, patients with prior CABG had more bleeding events, particularly more major bleeding events than those without prior CABG. However, ticagrelor monotherapy reduced bleeding events in both groups compared with DAPT. This suggests that whilst patients with prior CABG are at increased risk of ischaemic and bleeding events post PCI, ticagrelor monotherapy reduces bleeding risk without a concomitant increase in ischaemic risk.
The authors carried out an exploratory analysis to assess the effects of monotherapy versus DAPT on graft versus native vessel targets with no differences in treatment effects demonstrated. Whilst this is potentially interesting data, all patients underwent native vessel PCI with 4.3 % undergoing additional arterial graft PCI and 18.8 % undergoing additional vein graft PCI. Given the known limitations with SVG-PCI current recommendations support native vessel PCI where feasible5, the authors do not comment if the patients who underwent vein graft PCI underwent native vessel PCI to the same or a different territory.
This post-hoc analysis provides important hypothesis-generating data to support the discontinuation of aspirin at three months in patients with prior CABG undergoing PCI, however there are a number of important limitations that must be borne in mind. Firstly, by nature of the trial design patients who had an event prior to three months post PCI will not have been enrolled in this study. Therefore this strategy, presently, should only be considered in patients who are event-free at three months. Secondly, there are a number of inherent limitations to the post-hoc nature of the analysis including the fact that the relatively small sample size means that it was not possible to draw conclusions with regard to rare but clinically important differences in some adverse events. Furthermore, data regarding CABG such as time from initial surgery and bypass grafts was not available. Finally, the benefit of ticagrelor monotherapy cannot be extrapolated to suggest monotherapy with any P2Y12 inhibitor post PCI will produce the same results.
Historically, the focus within the PCI community has been on the ischaemic risk post PCI, however it is now understood that bleeding carries important morbidity and mortality therefore minimising bleeding without increasing ischaemic risk is a careful balance.
Overall, this study suggests that ticagrelor monotherapy can be considered at three months post PCI in patients both with and without CABG as a strategy to reduce bleeding risk without a concomitant increase in ischaemic risk. Further research is required to understand the optimal antiplatelet strategy in this population, in the interim clinicians must carefully assess individual patients bleeding and ischaemic risks when prescribing and reviewing antiplatelet therapy.
References
- Contemporary coronary artery bypass graft surgery and subsequent percutaneous revascularization. Nat Rev Cardiol. 2022 Mar;19(3):195–208.
- Brilakis ES, Rao SV, Banerjee S, Goldman S, Shunk KA, Holmes DR, et al. Percutaneous Coronary Intervention in Native Arteries Versus Bypass Grafts in Prior Coronary Artery Bypass Grafting Patients: A Report From the National Cardiovascular Data Registry. JACC: Cardiovascular Interventions. 2011 Aug 1;4(8):844–50.
- Welsh RC, Granger CB, Westerhout CM, Blankenship JC, Holmes DRJ, O’Neill WW, et al. Prior coronary artery bypass graft patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. JACC Cardiovasc Interv. 2010 Mar;3(3):343–51.
- Rathod KS, Beirne A, Bogle R, Firoozi S, Lim P, Hill J, et al. Prior Coronary Artery Bypass Graft Surgery and Outcome After Percutaneous Coronary Intervention: An Observational Study From the Pan‐London Percutaneous Coronary Intervention Registry. Journal of the American Heart Association. 2020 Jun 16;9(12):e014409.
- Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. European Heart Journal. 2019 Jan 7;40(2):87–165.




No comments yet!