Ultrathin, biodegradable-polymer sirolimus-eluting stent vs thin, durable-polymer everolimus-eluting stent
Selected in JACC: Cardiovascular Interventions by N. Ryan
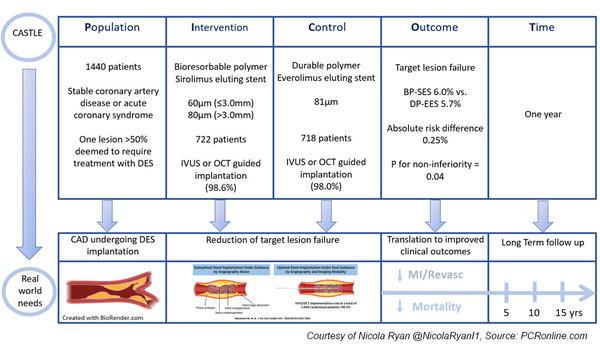
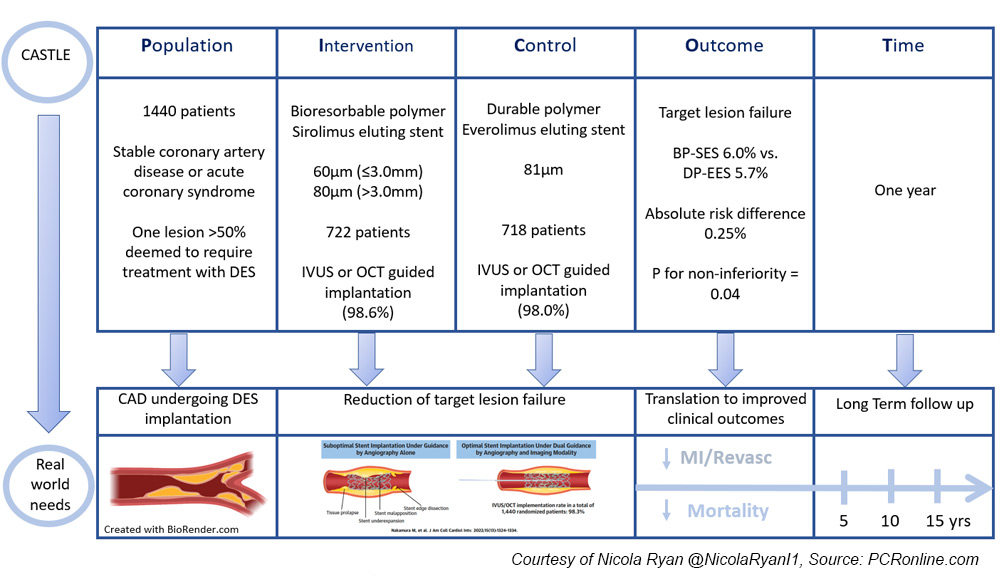
The CASTLE trial is a randomised multicentre prospective non-inferiority trial comparing ultra-thin strut bioresorbable polymer and thin strut durable polymer drug eluting stents implanted with intracoronary imaging guidance.
References
Authors
Masato Nakamura, Kazushige Kadota, Yoshihisa Nakagawa, Kengo Tanabe, Yoshiaki Ito, Tetsuya Amano, Yuichiro Maekawa, Akihiko Takahashi, Nobuo Shiode, Yoritaka Otsuka, Tomohiro Kawasaki, Yutaka Hikichi, Junya Shite, Ken Kozuma, Raisuke Iijima, Yoshitaka Murakami, and on behalf of the CASTLE Investigators
Reference
J Am Coll Cardiol Intv. 2022 Jul, 15 (13) 1324–1334
Published
July 2022
Link
Read the abstract
Reviewer
Latest contributions
Balloon lithotripsy added to conventional preparation before stent implantation in severely calcified coronary lesions Impact of pullback pressure gradient on clinical outcomes after percutaneous coronary interventions Validation of intravascular ultrasound-defined optimal stent expansion criteria for favorable 1-year clinical outcomesMy Comment
Why this study – the rationale/objective?
Drug eluting stents (DES) are currently the gold standard for patients undergoing percutaneous coronary intervention, however, there remains a not insignificant rate of device-orientated clinical endpoints (DOCE), 2-3 % per year.
Advances in stent design, one of which is the use of ultrathin strut stents, aim to reduce DOCE. Ultrathin strut stents have been shown to be superior in terms of TLF compared to thin strut stents1,2.
On the other hand, procedural aspects such as suboptimal stent implantation may also influence clinical outcomes. DES implantation guided by intracoronary imaging (IVUS or OCT) has been shown to be superior to angiography-guided DES implantation in terms of stent restenosis and thrombosis3,4.
Stent implantation guided by intracoronary imaging may help delineate the contribution of stent strut thickness to clinical events.
How was it executed? - the methodology
Patients undergoing angiography for chronic stable angina or acute coronary syndrome with one lesion > 50 % deemed to require treatment with DES were eligible for inclusion.
The use of either OCT or IVUS was left to the discretion of the operator.
Imaging-defined optimal stent implantation criteria were not specified in the trial.
Patients were randomised to treatment with a bioresorbable polymer sirolimus eluting stent (BP-SES) with a strut thickness of 60 µm (≥ 3.0 mm) and 80 µm (> 3.0 mm) or a durable polymer everolimus-eluting stent (DP-EES) with a strut thickness of 81 µm.
- The primary endpoint was target lesion failure (TLF) at one year a composite of cardiac death, target vessel MI and clinically driven target vessel revascularisation.
- Secondary endpoints included patient-orientated clinical endpoint, a composite of all-cause death, all MI and all revascularisation, the components of TLF, and stent thrombosis at one year after index PCI.
What is the main result?
Overall, between May 2019 and March 2020, 1,440 patients were included in the trial, 722 randomised to BP-SES, and 718 to DP-EES.
The majority presented with stable coronary artery disease (85 %), one fifth were female with a high prevalence of traditional CVRFs. Three quarters of treated lesions were complex, AHA B2/C, with one third bifurcations and 50 % LAD lesions.
OCT or IVUS guided stent implantation occurred in 98.6 % and 98 % of the BP-SES and DP-EES groups respectively.
- BP-SES was non-inferior to DP-EES in terms of TLF at one year. (BP-SES 6.0 % vs. DP-EES 5.7 %, absolute risk difference 0.25 %, p for non-inferiority = 0.04, p for superiority 0.845)
- There were no significant differences in the components of the primary endpoint between the groups, [Cardiac death (BP-SES 0.8 % vs. DP-EES 1.0 %, p = 0.7733), TVMI (BP-SES 4.3 % vs DP-EES 33.9 %, p = 0.706), clinically driven TV revascularisation (BP-SES 0.8 % vs. DP-EES 1.0 %, p = 0.773).
- The rates of POCE were similar between groups (BP-SES 8.9 % vs DP-EES 9.6 %, p = 0.625)
- Stent thrombosis rates were low with one definite or probable stent thrombosis in the BP-SES group with three possible stent thrombosis in the BP-SES and five in the DP-EES groups.
Critical reading and the relevance for clinical practice
The results of this study show that when guided by intra-coronary imaging, BP-SES were non-inferior to DP-EES in terms of TLF at one year.
Multiple factors influence the outcomes after implantation of DES, including technical factors related to both implantation and stent design, clinical presentation, lesion characteristics, and medical therapy.
Disentangling the individual contributions of each component can be difficult and, in this study, the authors attempted to assess the contribution of stent strut thickness to target lesion failure. This in and of itself is challenging, as a number of stent design factors must be considered.
Firstly, stent strut thickness is only one aspect of the stent design and the polymers used also differ between stents. A potential advantage of the bioresorbable polymer is that, after drug elution, the polymer dissolves, leaving behind a bare-metal stent; thus, the local inflammatory reaction and consequent increased risk of stent thrombosis is reduced compared to a durable polymer. Given that the follow-up is only one year, the polymer will not have completely dissolved in the BP-SES group; therefore longer-term outcomes will be of interest to see if this translates into improved outcomes in this group.
Secondly, relatively small numbers of patients with ACS were included in this trial, this subgroup may potentially benefit more from the bioresorbable polymer design; however, this was not demonstrated in the subgroup analysis, which will be limited by the subgroup size.
Finally, approximately a third of patients in the BP-SES group had stent diameters > 3.0 mm implanted where the stent thickness of 80 µm is almost identical to the DP-EES stent thickness of 81 µm.
The high rate, > 98 %, of intracoronary imaging use may have mitigated for differences between stent design. This remains hypothetical, given that the study did not randomise to angiography versus intracoronary imaging guidance. Furthermore, the study did not prespecify optimal stent implantation criteria, nor was core lab analysis used to assess intra-coronary imaging. It would have been of interest to compare the outcomes in patients with imaging-defined optimal stent implantation versus those not meeting imaging-defined optimal stent implantation criteria. The routine use of imaging in Japan is very high, therefore extrapolation to operators who are less facile with intracoronary imaging in settings where intracoronary imaging is routinely may not produce similar results.
The pre-specified three-year follow-up of the CASTLE study will be of interest, particularly given that the bioresorbable polymer will have completely degraded at that point. Given the numerous factors contributing to target lesion failure, it is increasingly difficult to disentangle each component. Improving clinical outcomes post PCI will undoubtedly require optimal PCI, a combination of appropriate imaging, plaque preparation, stent platform, stent optimization, and optimal medical therapy.
This study provides further support for the safety and efficacy of BP-SES allowing operators increased choice of stent platform.
References
- Kandzari DE, Mauri L, Koolen JJ, Massaro JM, Doros G, Garcia-Garcia HM, et al. Ultrathin, bioresorbable polymer sirolimus-eluting stents versus thin, durable polymer everolimus-eluting stents in patients undergoing coronary revascularisation (BIOFLOW V): a randomised trial. Lancet. 2017 Oct 21;390(10105):1843–52.
- Iglesias JF, Muller O, Heg D, Roffi M, Kurz DJ, Moarof I, et al. Biodegradable polymer sirolimus-eluting stents versus durable polymer everolimus-eluting stents in patients with ST-segment elevation myocardial infarction (BIOSTEMI): a single-blind, prospective, randomised superiority trial. Lancet. 2019 Oct 5;394(10205):1243–53.
- Shin DH, Hong SJ, Mintz GS, Kim JS, Kim BK, Ko YG, et al. Effects of Intravascular Ultrasound–Guided Versus Angiography-Guided New-Generation Drug-Eluting Stent Implantation: Meta-Analysis With Individual Patient–Level Data From 2,345 Randomized Patients. JACC: Cardiovascular Interventions. 2016 Nov 14;9(21):2232–9.
- Zhang J, Gao X, Kan J, Ge Z, Han L, Lu S, et al. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: The ULTIMATE Trial. Journal of the American College of Cardiology. 2018 Dec 18;72(24):3126–37.




No comments yet!