A randomised trial of sirolimus- versus paclitaxel-coated balloons for de novo coronary lesions
Selected in EuroIntervention Journal by M. Gitto , N. Ryan
The present study compares a novel sirolimus-coated balloon with a clinically proven paclitaxel-coated balloon for the treatment of de novo coronary lesions, demonstrating non-inferiority in terms of angiographic late lumen loss at 6 months.
References
Authors
Scheller Bruno, Mangner Norman, Jeger Raban, Afan Samuel, Mahfoud Felix, Woitek Felix, Fahrni Gregor, Schwenke Carsten, Schnorr Beatrix, Kleber Franz
Reference
DOI: 10.4244/EIJ-D-23-00868
Published
Nov 4, 2024
Link
Read the abstractReviewers
Our Comment
Designed by Mauro Gitto, Nicola Ryan - Source: PCRonline.com
Why this study – the rationale/objective?
Drug-coated balloons (DCBs) are increasingly used for the treatment of de novo coronary lesions, to avoid stent implantation or to reduce stent length.
DCBs do not provide a class effect, and their efficacy varies based on both the eluted antiproliferative agent and the excipient.
This trial aimed to test a new highly crystalline sirolimus-coated balloon (SCB) with butylated hydroxytoluene as the excipitant for the treatment of de novo coronary artery disease.
How was it executed – the methodology?
Patients with stable or unstable angina and de novo coronary lesions (> 70 % stenosis or 50-70 % stenosis with positive functional assessment) were eligible for enrollment in four German and Swiss centres.
After successful lesion preparation, patients were randomised to receive DCB angioplasty, with either the SeQuent SCB [B. Braun] or the SeQuent Please NEO paclitaxel-coated balloon (PCB, [B. Braun]).
All participants underwent angiographic follow-up at 6 months.
What is the main result?
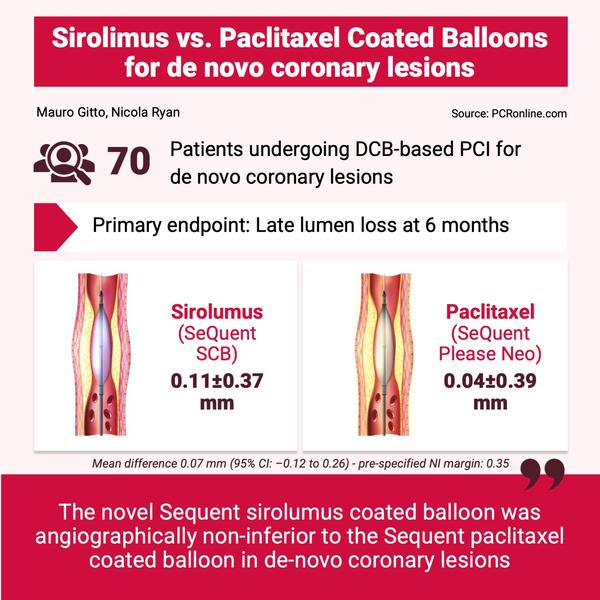
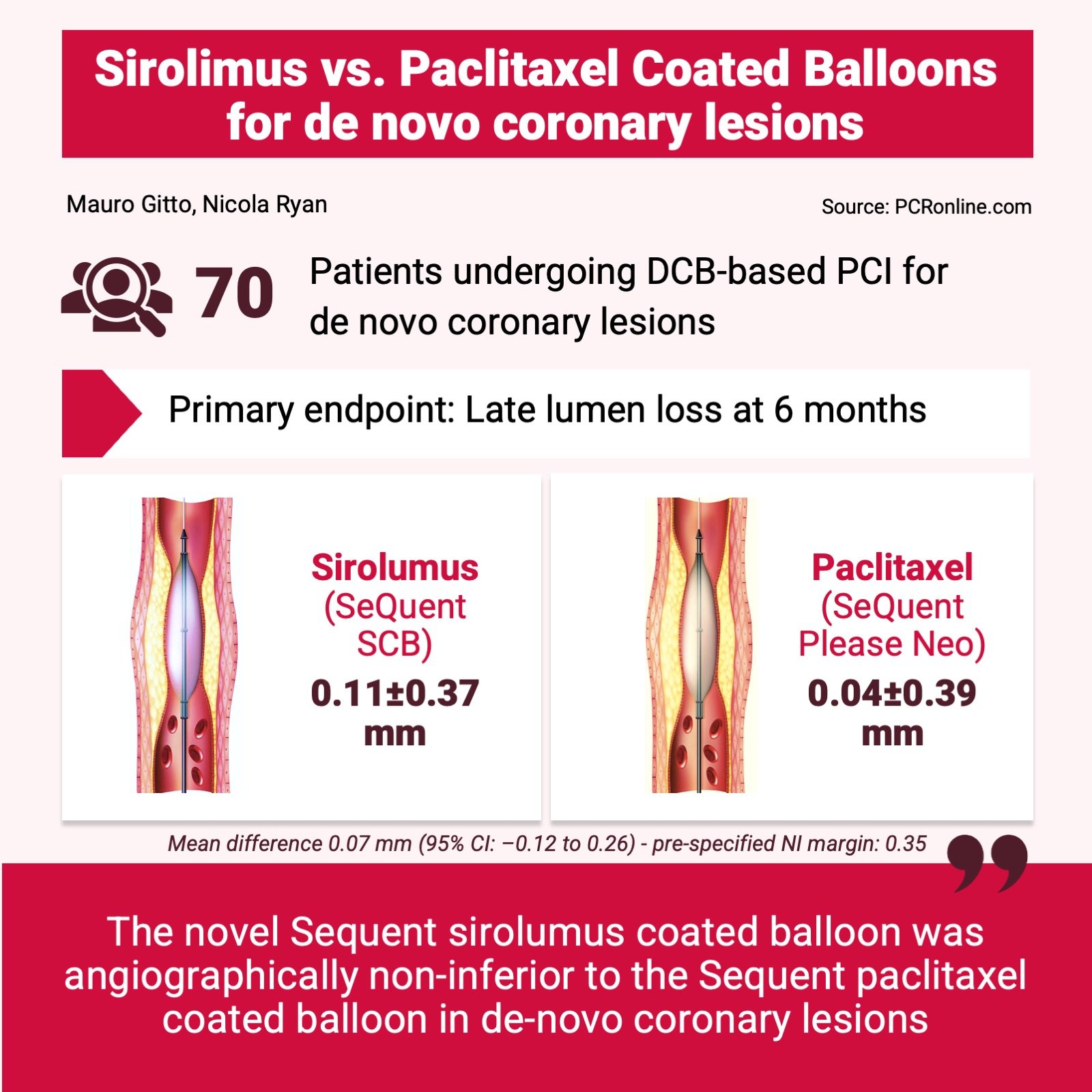
From February 2019 to February 2022, 70 patients with de novo coronary lesions were enrolled. The majority of patients were male, with a mean age of 67 years old. Three patients required bailout DES implantation (1 in the SCB group and 2 in the PCB group).
- At 6 months, SCB was non-inferior to PCB, with a mean LLL of 0.11 ± 0.37 mm in the SCB group, and 0.04 ± 0.39 mm in the PCB group. The mean difference of 0.07 mm (95 % CI: -0.12 to 0.26 mm) met the pre-specified non-inferiority margin of 0.35 mm.
- Late lumen enlargement was more common in the PCB group, but the difference did not reach statistical significance (56 % vs. 44 %, p = 0.544).
Critical reading and the relevance for clinical practice
This trial demonstrated that the SeQuent SCB was non-inferior to the SeQuent Please NEO PCB in terms of LLL at 6 months.
While PCBs represent the most widely used DCBs, an increasing number of SCBs are being introduced to the market. Unlike paclitaxel, sirolimus has a cytostatic mechanism of action and a poor lipophilicity, making its tissue uptake highly dependent on the excipient. As a result, assessing the safety and angiographic efficacy of newly introduced SCBs is crucial.
Most of the included lesions were located in large vessels, with a mean reference vessel diameter of 2.95 mm. Despite this, the LLL was very low in both groups, and three-fold lower than the pre-specified non-inferiority margin (0.35 mm) in the SCB group. The relatively wide non-inferiority margin may have resulted in the study being underpowered to detect small differences in terms of LLL, the clinical relevance of which does however remain uncertain. Similarly, the absence of significant differences in late lumen enlargement, which is typically more pronounced with PCBs, raises additional concerns about the potential lack of power for angiographic endpoints.
The study results align with those of a prior Malaysian study using the same SCB with regard to LLL; however, the PCB was associated with a significant late lumen enlargement in that study. Conversely, the study findings differ from the TRANSFORM I trial, which compared a SCB with different drug delivery technology to the SeQuent Please NEO PCB. These differences highlight the importance of device-comparing studies in DCB research, as a class effect cannot be assumed across different DCBs.
Finally, while the angiographic outcomes suggest similar efficacy between the investigated SCB and PCB, further studies are needed to confirm whether these translate into comparable clinical benefits.







No comments yet!