PACMAN AMI: Effects of Alirocumab on Coronary Atherosclerosis Assessed by Serial Multimodality Intracoronary Imaging in Patients With Acute Myocardial Infarction
Reported from ACC 2022
Nicola Ryan provides a PICOT analysis of PACMAN AMI: a double-blind, placebo-controlled, randomized trial presented at ACC 2022 and simultaneously published in JAMA.
The PACMAN-AMI Trial is a prospective randomised, multicentre, trial comparing the effect of early administration of biweekly subcutaneous PCSK9 inhibitor alirocumab versus placebo on IVUS-derived percent atheroma volume (PAV) in patients undergoing PCI for acute myocardial infarctions.
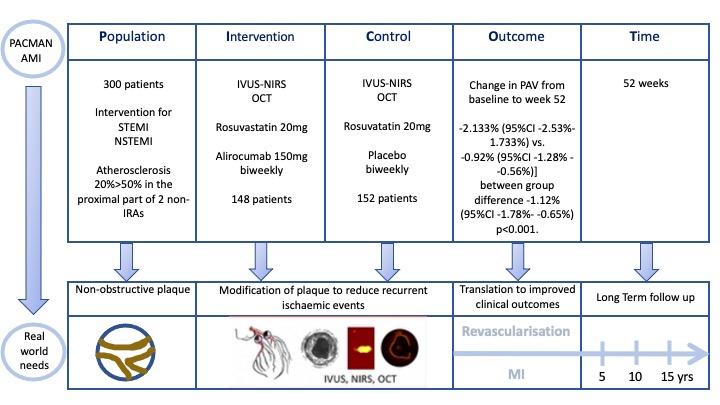
PICOT analysis of the PACMAN-AMI trial courtesy of Nicola Ryan
Why this study – the rationale/objective?
Several plaque characteristics identifiable by intracoronary imaging, such as large atheroma volume as assessed by IVUS, large lipid burden as assessed by NIRS and OCT assessed thin caps, affect the likelihood of a plaque to progress or lead to an acute coronary event.
Addition of PCSK9 inhibitors to statin therapy in patients with elevated LDL-C has been shown to reduce cardiovascular events. Patients with AMI are particularly at risk of recurrent events due to the presence of high-risk non-obstructive plaques in the non-infarct related arteries. Therefore modification of plaque composition with early administration of alirocumab would be of interest in this group.
How was it executed - the methodology?
Patients undergoing PCI for STEMI or NSTEMI with angiographic evidence of atherosclerosis >20% and <50% stenosis by visual assessment in the proximal part of two non IRAs were eligible for inclusion. An LDL-C of at least 125mg/dL if not on stable statin dose for at least 4 weeks or at least 70mg/dL if on a stable statin dose for at least 4 weeks. All eligible patients underwent intracoronary imaging with a combined NIRS-IVUS catheter and OCT of the two non-IRA’s and were then randomised to alirocumab 150mg or placebo via subcutaneous injection biweekly for 52 weeks. All patients received rosuvastatin 20mg daily.
- The primary outcome was change in percentage atheroma volume (PAV) from baseline to week 52
- Powered secondary endpoints were change in maximum lipid core burden index within 4mm via NIRS and change in minimal fibrous cap thickness (FCT) via OCT from baseline to week 52
- Secondary imaging endpoints were normalised total atheroma volume via IVUS, change in total LCBI via NIRS and changes in mean FCT and in mean angular extension of macrophages via OCT
- Secondary non-imaging related endpoints included the incidence of adjudicated events (all-cause mortality, cardiac death, myocardial infarction, ischaemia driven coronary revascularisation and stroke or TIA) and adverse events and changes in biomarkers.
What is the main result?
Overall 300 patients were included in the trial, 52.7% presenting with STEMI 47.3% with NSTEMI, the majority were male in their 50’s with a high prevalence of traditional CVRFs. Randomisation to alirocumab occurred in 148 and placebo in 152, in both groups the rates of both prior statin (11.5% vs. 13.2%) and high intensity statin (7.4% vs. 5.9%) use was low prior to enrolment.
- There was a significantly greater reduction in PAV in the alirocumab group compared to the placebo [-2.133% (95%CI -2.53% - 1.733%) vs. -0.92% (95%CI -1.28% - -0.56%)] between group difference -1.12% (95%CI -1.78% - -0.65%) p<0.001.
- The change in maximum lipid core burden index within 4mm had a significantly greater reduction in the alirocumab group (−79.42 vs. −37.60; between-group difference, −41.24 (95%CI −70.71 - −11.77, p=0.006)
- There was a greater increase in the change in mean minimal FCT in the alirocumab group [62.67 μm (95% CI 48.84-76.50)] compared to the placebo group [33.19 μm (95%CI, 22.22-44.16)] between-group difference, [29.65 μm (95%CI 11.75-47.55); p=0.001.]
- All other secondary imaging outcomes showed a significant difference in favour of alirocumab
- Ischaemia-driven revascularisation rates were lower in the alirocumab group (4.8% vs. 11% p=0.04) with no other difference in clinical events.
- Overall adverse event rates were low in both groups with general allergic reaction higher in the alirocumab group (3.4% vs. 0.0%, p=0.03)
- Baseline mean LDL-C was 152.8 ± 33.8 mg/dl with week 52 LDL-C 74.4 ± 30.5 mg/dL in the placebo and 23.6 ± 23.8 mg/dL in the alirocumab group (p<0.001) with a between group difference -54.7mg/dL (95%CI -63.5 - -45.9, p<0.001).
Critical reading and the relevance for clinical practice
The results of this study show that the early addition of alirocumab to high-intensity statin therapy in patients presenting with AMI leads to greater plaque regression, as demonstrated by a greater decrease in PAV, larger reduction in lipid burden and greater increase in fibrous cap thickness in non-infarct related arteries compared to placebo after 52 weeks of treatment. Compared to prior trials, this trial showed a larger extent of PAV regression (2.13%), likely due to the low numbers of patients established on statin therapy at presentation and the extent of PAV regression approximates the combined PAV regression seen with statin therapy and additional or PCSK9 inhibitor to statin therapy.
This trial is unique in providing a comprehensive assessment of plaque morphology, composition and burden by using a combination of IVUS-NIRS and OCT catheters in both non-IRAs. These findings provide a mechanistic rationale for early addition of alirocumab to high-intensity statins in patients presenting with AMI in order to stabilise plaques, reduce the lipid core and induce plaque regression. It must be borne in mind that there are a number of limitations to this trial including the relatively small numbers of patients included and the fact that 12% of patients did not undergo assessment at 52 weeks. The plaque characteristics of these patients may be different to those included. Whilst this study shows that the early initiation of alirocumab appears safe with low rates of adverse clinical and safety events it was not powered to determine clinical outcomes. The question remains whether these improvements in plaque characteristics with early initiation of alirocumab translate into improved clinical outcomes in patients with AMI.




No comments yet!