BIOFLOW DAPT - Biodegradable-polymer or durable-polymer stents in patients at high bleeding risk: A randomized, open-label clinical trial
Reported from ESC Congress 2023
Nicola Ryan reports on the BIOFLOW DAPT trial results presented during the ESC 2023 Congress in Amsterdam by Marco Valgimigli and published in Circulation.
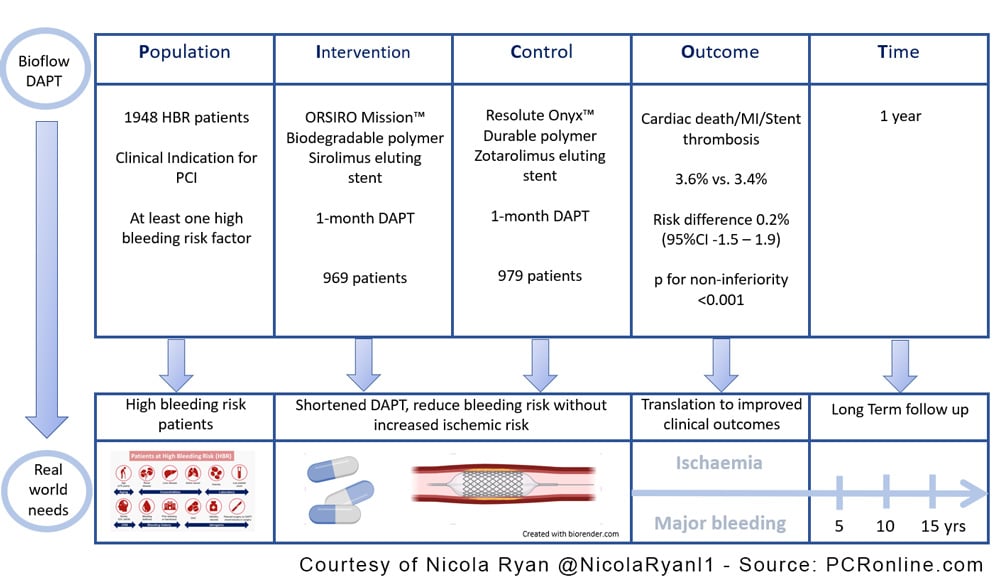
BIOFLOW-DAPT is an open-label randomised trial comparing the safety and efficacy of a biodegradable-polymer sirolimus-eluting stent with a durable-polymer zotarolimus-eluting stent in high-bleeding risk patients receiving one month of DAPT after undergoing PCI.

PICOT analysis of BIOFLOW DAPT - Courtesy of Nicola Ryan @NicolaRyanl1 - Source: PCRonline.com
Why this study – the rationale/objective?
Prior studies have established that PCI with DES and one month of DAPT is a safe and efficacious strategy in high-bleeding risk patients (1). To date, the comparison between stent platforms has been limited with the majority of trials comparing DES to BMS, with Onyx One comparing a durable-polymer zotarolimus-coated stent with a polymer-free umirolimus-coated stent (2). This trial compares a biodegradable-polymer sirolimus-eluting stent with a durable-polymer zotarolimus-eluting stent in high-bleeding risk patients receiving one month of DAPT post-PCI.
How was it executed - the methodology?
Patients undergoing PCI for acute or chronic coronary syndrome with at least one high bleeding risk criteria were eligible for inclusion. Patients were randomised 1:1 to a biodegradable-polymer sirolimus-eluting stent or a durable-polymer zotarolimus-eluting stent with DAPT for one month. Patients were assessed at one month to ensure they remained eligible for DAPT discontinuation (no adverse events and compliant with therapy), SAPT with aspirin or P2Y12 inhibitor was at the discretion of the treating physician however patients were stratified by SAPT drug. Patients underwent clinical follow-up at 30 days, 3 and 12 months.
- The primary outcome was a composite of cardiac death, MI, definite or probable stent thrombosis.
- Secondary endpoints included the components of the primary endpoint, MACCE (death, MI or stroke), MACE (death, MI or TVR), stroke, target vessel or lesion failure, bleeding events.
What is the main result?
Overall, between February 2020 and September 2021, 1948 patients were randomised; 969 were assigned to biodegradable-polymer sirolimus-eluting stent (952 received at least one stent) and 979 were assigned to durable-polymer zotarolimus-eluting stent (969 received at least one stent). The majority of patients were male (68.6%) with traditional CVRF common. Age ≥75 was the most frequent HBR criteria with ≥2 HBR criteria in over half the population (1040 patients). There were relatively few patients with STEMI (1.7%) included in the trial with the majority of presentations chronic coronary syndrome. Compliance with DAPT discontinuation was high with aspirin the SAPT in approximately 40%.
- There was no difference in the primary outcome 3.6% in the biodegradable-polymer sirolimus-eluting stent group versus 3.4% in the durable-polymer zotarolimus-eluting stent (Risk difference 0.2%, 95%CI -1.5 – 1.9, p for non-inferiority <0.001).
- There were no differences in MACCE (6.6% vs. 5.6%, HR 1.18, 95%CI 0.81-1.70), MI (1.2% vs. 1.1%, HR 1.11 95%CI 0.49-2.50), target vessel failure (4.7% vs. 5.8%, HR 0.83 95%CI 0.56-1.23).
- There were no differences in the overall bleeding events between groups (BARC 1-5 16.1% vs 13.0% HR 1.24 95% CI 0.98–1.58), with low rates of BARC 3 or 5 bleeding (3.55 vs. 3.8%, HR 0.91, 95% CI 0.56-1.46)
Critical reading and the relevance for clinical practice
The results of this study show that PCI with a biodegradable-polymer sirolimus-eluting stent is non-inferior to PCI with a durable-polymer zotarolimus-eluting stent in high-bleeding risk patients receiving one month of DAPT followed by SAPT. This trial is of interest to the community as high bleeding risk patients are frequently encountered in clinical practice with increasing life expectancy as well as increased complexity of patients referred for PCI. Increasing the evidence with regard to the choice of stent platforms in this population allows flexibility in daily clinical practice as well as allows clinicians to confidently abbreviate DAPT in carefully selected HBR patients. Of note this study only includes patients who were event-free and compliant with medications at one month review, therefore this is a strategy that can only be applied following clinical review at one month.
There were no significant differences in the primary or secondary endpoints using both the ARC-2 criteria for MI as well as the third universal definition, as expected events were almost twice as high when the third universal definition of the MI was used however did not differ between groups. Importantly the non-inferiority persisted when analysed in the per-protocol population.
Landmark analysis at thirty days did not show difference in the primary outcomes between the groups though there was reduced target vessel failure in the biodegradable-polymer sirolimus-eluting stent (2.8% vs 4.6%, HR 0.61, 95% CI 0.37-0.99). Reduced rates of TV MI and stent thrombosis have been seen in all-comer trials of the biodegradable-polymer sirolimus-eluting stent (3). Overall event rates were lower than expected in the population some of which may be explained by lack of an angiographic core lab however likely represents real-world clinical practice.
It must be borne in mind that there are a number of limitations to this trial including the fact that physicians were at liberty to choose the SAPT. Whilst there was stratification for this, prior studies have suggested that SAPT with the P2Y12 inhibitor ticagrelor is preferable to SAPT with aspirin, though patients had completed three months of DAPT in this study (4). There were relatively low numbers of patients with STEMI included in this trial which may be explained by the requirement for assessment of the LV function prior to randomisation, which is not always carried out in this population. The STEMI population is one that it would be of particular interest to include in this trial as due to the acuity of the presentation it is not always possible to fully evaluate bleeding risk prior to stent implantation.
Whilst this study was not designed to support one month of DAPT in all-comers, it increases the choice of stent platforms with evidence supporting one month of DAPT in HBR patients. As always, decisions with regard to ischaemic and bleeding risk need to be carefully assessed and individualised for each patient.
Latest news from ESC Congress 2023
References
- Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrié D, Naber C, et al. Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. N Engl J Med. 2015 Nov 19;373(21):2038–47.
- Windecker S, Latib A, Kedhi E, Kirtane AJ, Kandzari DE, Mehran R, et al. Polymer-based or Polymer-free Stents in Patients at High Bleeding Risk. New England Journal of Medicine. 2020 Feb 12;0(0):null.
- Kandzari DE, Koolen JJ, Doros G, Garcia-Garcia HM, Bennett J, Roguin A, et al. Ultrathin Bioresorbable Polymer Sirolimus-Eluting Stents Versus Durable Polymer Everolimus-Eluting Stents: BIOFLOW V Final 5-Year Outcomes. JACC Cardiovasc Interv. 2022 Sep 26;15(18):1852–60.
- Escaned J, Cao D, Baber U, Nicolas J, Sartori S, Zhang Z, et al. Ticagrelor monotherapy in patients at high bleeding risk undergoing percutaneous coronary intervention: TWILIGHT-HBR. European Heart Journal [Internet]. 2021 Oct 18 [cited 2021 Nov 2];(ehab702). Available from: https://doi.org/10.1093/eurheartj/ehab702






No comments yet!