29 Aug 2023
Extended clopidogrel monotherapy versus DAPT in high-risk patients: The OPT-BIRISK trial
Reported from ESC Congress 2023
Nicola Ryan provides her take on the final results of OPT-BIRISK which were presented by Yaling Han during the ESC 2023 congress in Amsterdam.
Note the assumptions in this article are based on the presentation alone as the trial has not yet been published.
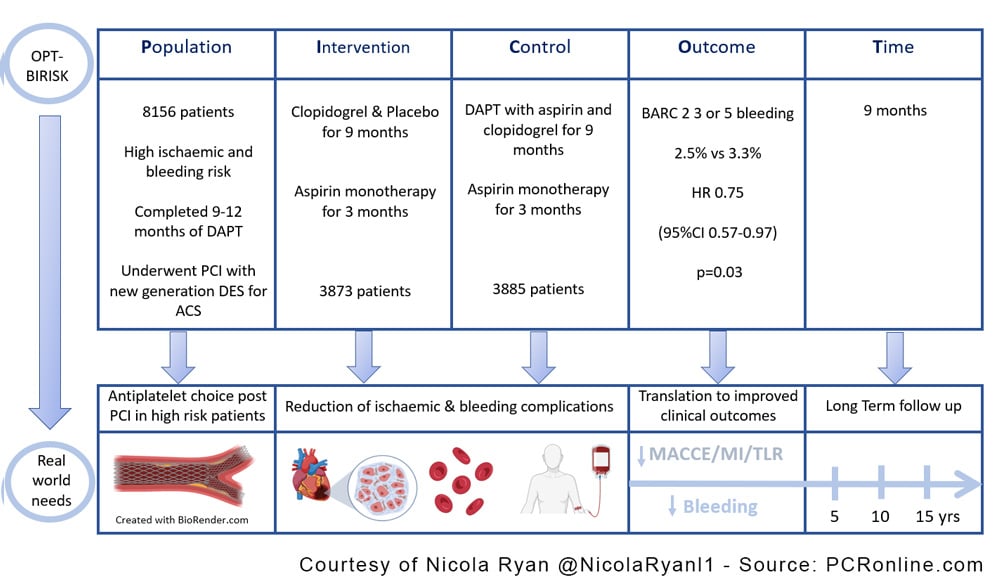
The OPT-BIRISK trial is a multicentre, double-blind, placebo-controlled superiority trial comparing clopidogrel monotherapy with DAPT, aspirin and clopidogrel, in patients with both high bleeding and ischaemic risk who have completed 9-12 months of DAPT post PCI for ACS.

PICOT analysis of OPT-BIRISK - Courtesy of Nicola Ryan @NicolaRyanl1 - Source: PCRonline.com
Why this study – the rationale/objective?
Antiplatelet therapy post PCI aims to reduce ischaemic events however, comes with the concomitant increased risk of bleeding. It is recognised that the risk factors for both bleeding and ischaemic events can overlap therefore the optimal strategy to reduce both ischaemic and bleeding events in this population is of interest. Typically, following a period of dual antiplatelet therapy, aspirin monotherapy is prescribed lifelong to reduce the ischaemic risk. More recent trials (1,2) have suggested that P2Y12 monotherapy may be preferable in terms of reducing bleeding risk without increasing ischaemic risk.
How was it executed - the methodology?
Patients who presented with an ACS and underwent PCI with a DES were eligible for randomisation 9-12 months post PCI provided they had been compliant with DAPT and had no significant adverse events in the previous six months. Inclusion criteria varied by age with patients <65 years required to have both an ischaemic and bleeding risk factor, aged 65-75 could have either a bleeding or ischaemic risk factor and ≥75 years were eligible based on age alone. Patients were randomised in a 1:1 fashion to clopidogrel and placebo for 9 months or clopidogrel and aspirin for 9 months, both groups completed 3 months of aspirin after this.
- The primary endpoint was BARC 2, 3 or 5 bleeding
- The key secondary endpoint was MACCE a composite of all-cause mortality, MI, stroke or clinically driven revascularisation
- Other endpoints included the components of MACCE, any bleeding and stent thrombosis.
What is the main result?
Overall from February 2018 to December 2020, 7758 patients were included, 3872 to clopidogrel and placebo and 3885 to clopidogrel and aspirin. Overall loss to follow-up and protocol violations were similar amongst both arms. A little over 40% of the population were female with a mean age of 64 years, 60% presented with unstable angina with diabetes (52%) and prior ischaemic stroke (15%) common. The majority of lesions were in the LAD with a quarter of patients presenting with small vessel disease and >80% with diffuse disease, with a mean stent length of 47mm. Patients had a mean of 3.2±1.6 high ischaemic risk criteria and 1.4±0.9 high bleeding risk criteria.
- Clopidogrel monotherapy was superior to DAPT in terms of the primary endpoint of BARC 2,3 or 5 bleeding at 9 months, 2.5% vs. 3.3%, (HR 0.75, 95%CI 0.57-0.97, p=0.03).
- MACCE was significantly lower in the clopidogrel monotherapy arm 2.6%. vs 3.5%, (HR 0.74, 95%CI 0.57-0.96, p=0.02).
Critical reading and the relevance for clinical practice
The results of this trial show that in patients with ACS with both high bleeding and ischaemic risks, who complete 9-12 months of DAPT, clopidogrel monotherapy was superior to clopidogrel and aspirin in reducing bleeding. Though not powered for superiority MACCE was also reduced in the clopidogrel monotherapy arm. The benefit of reduced bleeding was driven by reduced BARC 2 bleeds with similar rates of BARC 3 & 5 bleeding between groups. Interestingly the authors used different eligibility criteria for differing age groups.
Whilst the pre-specified subgroup analysis did not show differences between the age groups, it will be interesting to understand the breakdown of ischaemic and bleeding risk criteria by age group particularly as 149 patients had no ischaemic and 934 had no bleeding risk criteria.
Until the full manuscript is published it is difficult to understand and interpret the true risk profile of these patients at the time of randomisation. Furthermore, in order to be included in the trial, patients who had a bleeding or ischaemic event whilst on DAPT were not eligible for inclusion therefore this strategy can only be generalised to patients who are event-free 9-12 months post PCI.
Some important limitations need to be borne in mind including the relative homogeneity of the population in which the study was conducted, all centres were in China therefore the generalisability to other populations needs to be considered. Clopidogrel non-responsiveness remains a concern due to the risk of stent thrombosis however there were no differences in stent thrombosis rates between groups and routine platelet reactivity testing is not recommended. The majority of patients included in this study presented with unstable angina which may influence the outcomes however given that patients were not randomised until 9-12 months post PCI the population was moving towards the chronic phase of the ACS/CCS spectrum.
Overall this trial adds further weight to the benefit of a strategy of P2Y12 monotherapy in patients with high bleeding and ischaemic events. Given that the follow-up time is only 9 months post-randomisation it will be of interest to understand the longer-term follow-up given that antiplatelet therapy is typically prescribed lifelong, though this population were switched to SAPT with aspirin at completion of the study period. To date the optimal long-term anti-platelet strategy remains undetermined however P2Y12 monotherapy appears to be a promising option in carefully selected patients. In real-world clinical practice clinicians must carefully assess and balance ischaemic and bleeding risks.
References
- Koo BK, Kang J, Park KW, Rhee TM, Yang HM, Won KB, et al. Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): an investigator-initiated, prospective, randomised, open-label, multicentre trial. Lancet. 2021 May 14;
- Baber U, Dangas G, Angiolillo DJ, Cohen DJ, Sharma SK, Nicolas J, et al. Ticagrelor alone vs. ticagrelor plus aspirin following percutaneous coronary intervention in patients with non-ST-segment elevation acute coronary syndromes: TWILIGHT-ACS. Eur Heart J. 2020 Oct 1;41(37):3533–45.
Latest news from ESC Congress 2023




1 comment
Thank you Nicola Question: taking into account the result of this trial ( and others…) what are your antiplatelet advice for a 82 years old mâle -without any past history of bleeding - moderate renal function impairement, presenting with NSTMI, who underwent PCI with 3.5 x 20 mm DES in the proximal LAD critical lesion ? Thank you for your answer Nicola