Fractional flow reserve-guided complete or culprit-only PCI in patients with ST-elevation myocardial Infarction - FULL REVASC
Reported from ACC.24
Nicola Ryan provides her take on the FULL REVASC trial which was presented at ACC.24 in Atlanta and published Simultaneously in NEJM.
The FULL REVASC trial is a multicentre registry-based randomised control trial comparing FFR-guided complete revascularisation of non-culprit lesions versus culprit lesion PCI alone in patients presenting with STEMI or very high-risk non-STEMI.
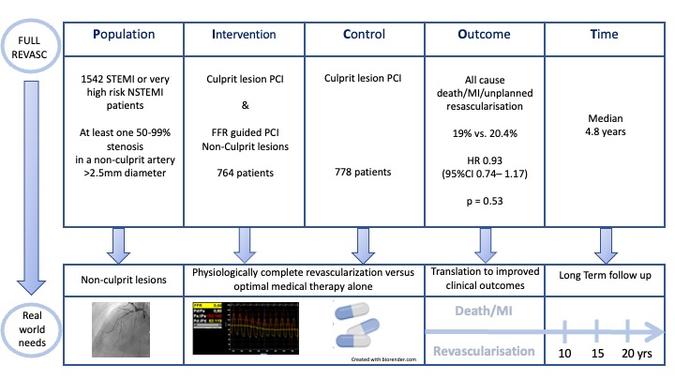
PICOT analysis of FULL REVASC - Courtesy of Nicola Ryan @NicolaRyanl1 - Source: PCRonline.com
Why this study – the rationale/objective?
Prior trials of FFR-guided complete revascularisation versus culprit-only PCI in all-comer STEMI patients (1,2) have shown a reduction in composite endpoints including all-cause death, MI and unplanned revascularisation however have not been powered to demonstrate a benefit in hard endpoints of death and MI alone. FULL REVASC was designed to evaluate FFR-guided complete revascularisation versus culprit-only PCI in patients presenting with STEMI or very high-risk NSTEMI. Of note, publication of the COMPLETE trial (3) led to early termination of recruitment and a change in the primary endpoint of the FULL REVASC trial.
How was it executed - the methodology?
Patients undergoing PCI for STEMI or urgent PCI for high-risk NSTEMI (ongoing chest pain, acute heart failure, dynamic ST-T wave changes, haemodynamic instability or life-threatening ventricular arrhythmias) were eligible for inclusion if they had at least one 50-99% stenosis in a non-culprit artery >2.5mm diameter. Patients with CTOs could be included if they had further non-culprit stenosis. LMS disease, prior CABG and cardiogenic shock were exclusion criteria. Patients were randomised 1:1 within 6 hours of the index procedure to FFR-guided revascularisation or culprit-only revascularisation. FFR-guided revascularisation was permitted during the index procedure or staged during the index hospitalisation in the FFR-guided group.
- The primary outcome was a composite of death from any cause, new myocardial infarction or unplanned revascularisation.
- The key secondary outcomes were a composite of death from any cause or myocardial infarction and unplanned revascularisation.
What is the main result?
Overall, between August 2016 and September 2019, 1542 patients were randomised; 764 assigned to FFR-guided complete revascularisation and 778 assigned to culprit-only revascularisation. The majority of patients were male (76%) with a mean age of 65 years and traditional CVRFs were common. The majority of patients (91%) presented with STEMI 45% with a culprit in the RCA and one-third with a culprit in the LAD. Non-culprit lesions were located in the LAD in over half the population. In the FFR-guided revascularisation group, complete revascularisation was carried out as a staged procedure in 78.8% at a median time of 2 days post initial PCI. FFR evaluation led to no further intervention in 40% of patients. Patients were followed up for a median of 4.8 years (IQR 4.3-5.2)
- There was no difference in the primary outcome at median follow-up of 4.8 years
- FFR guided 19% vs Culprit only 20.4% (HR 0.93, 95% CI 0.74-1.17, p =0.53).
- There were no differences in the composite of death from any cause or MI
- FFR guided 16.5% vs. Culprit only 15.3%, (HR 1.12, 95% CI 0.87-1.44)
- Unplanned revascularisation was similar between groups
- FFR guided 9.2% vs. Culprit only 11.7%, (HR 0.76, 95% CI 0.56-1.04)
Critical reading and the relevance for clinical practice
The results of this trial show that in patients presenting with a STEMI or very high-risk NSTEMI, FFR-guided complete revascularisation did not reduce the risk of a composite of death from any cause, new myocardial infarction or unplanned revascularisation at a median of 4.8 years follow up compared to culprit only PCI. This trial is at odds with the largest trial of complete versus culprit-only revascularisation published to date. In the COMPLETE trial (3) angiographically guided complete revascularisation was associated with a 26% reduced risk of cardiovascular death or MI at a median of three years follow-up.
Prior trials of FFR-guided complete revascularisation in all-comer STEMI patients have shown reduction in the primary endpoint with DANAMI-3-PRIMULTI (1) showing reduced all-cause mortality, non-fatal revascularisation and ischaemia-driven revascularisation in the FFR guided complete revascularisation arm and COMPARE-Acute (2) demonstrating a reduction in the composite of death from any cause, nonfatal myocardial infarction, revascularization, and cerebrovascular events at one year. Neither of these trials was powered for the endpoints of all-cause death or MI. The results of the largest prior trial of physiology-guided revascularisation the FIRE trial (4) also differ from the current results. The FIRE trial which included patients over the age of 75 with a STEMI or NSTEMI, demonstrated a 27% relative risk reduction of the composite of death, MI, stroke or ischaemia-driven revascularisation in the physiology-guided complete revascularisation arm compared to the culprit PCI alone. The FIRE trial also demonstrated a reduction in the composite of CV death or new MI (HR 0.64, 95% CI 0.47-0.88).
Of note the original primary outcome of the FULL REVASC trial was a composite of death from any cause or myocardial infarction at one year with a planned enrolment of 4052 patients. With the publication of the COMPLETE trial (3), recruitment to FULL REVASC was stopped early due to ethical and feasibility reasons. At the point of termination, 1542 patients had been randomised and the primary outcome was changed to include unplanned revascularisation.
Subgroup analysis of the primary endpoint did not demonstrate any differences, though a trend was seen for complete revascularisation being better in non-culprit lesions of 90-99% severity. This finding does not reach significance and may simply be play of chance however it fits with the finding in COMPLETE (3) of the benefit of complete revascularisation being driven by lesions >80% angiographic severity. Stent thrombosis, restenosis and target vessel revascularisation were numerically but not statistically higher in the FFR-guided revascularisation group, again this may be play of chance but highlights the non-benign nature of PCI and importance of optimising all interventions. The FFR-guided group had a longer length of stay, radiation time and contrast volume than the culprit-only group with no differences in safety outcomes of stroke, major bleeding and contrast-associated acute kidney injury.
To date, the unanswered question in complete versus culprit-only revascularisation is are their particular high-risk vulnerable plaque features in the non-culprit lesions of patients presenting with STEMI/NSTEMI which lead to improved outcomes with complete revascularisation. Certainly, the results of the recently published PREVENT trial (5) suggest that PCI in patients with vulnerable plaque characteristics in non-flow limiting lesions is beneficial. Given these findings the results of ongoing COMPLETE-2 trial and in particular the COMPLETE-2 OCT sub-study are awaited with interest (ClinicalTrials.gov number, NCT05701358).
Whilst FULL REVASC did not demonstrate a benefit to FFR-guided complete revascularisation in patients with STEMI or high-risk NSTEMI it is difficult to draw definitive conclusions from the results. In all patients decisions regarding complete or culprit-only revascularisation should be individualised taking into account not only the current evidence, but also the complexity/risk of the complete revascularisation as well as the patients individual co-morbidities and preferences.
References
- Engstrøm T, Kelbæk H, Helqvist S, Høfsten DE, Kløvgaard L, Holmvang L, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): an open-label, randomised controlled trial. The Lancet. 2015 Aug 15;386(9994):665–71.
- Smits PC, Abdel-Wahab M, Neumann FJ, Boxma-de Klerk BM, Lunde K, Schotborgh CE, et al. Fractional Flow Reserve–Guided Multivessel Angioplasty in Myocardial Infarction. New England Journal of Medicine. 2017 Mar 30;376(13):1234–44.
- Mehta SR, Wood DA, Storey RF, Mehran R, Bainey KR, Nguyen H, et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2019 Oct 10;381(15):1411–21.
- Biscaglia Simone, Guiducci Vincenzo, Escaned Javier, Moreno Raul, Lanzilotti Valerio, Santarelli Andrea, et al. Complete or Culprit-Only PCI in Older Patients with Myocardial Infarction. New England Journal of Medicine. 2023 Sep 6;389(10):889–98.
- Park SJ, Ahn JM, Kang DY, Yun SC, Ahn YK, Kim WJ, et al. Preventive percutaneous coronary intervention versus optimal medical therapy alone for the treatment of vulnerable atherosclerotic coronary plaques (PREVENT): a multicentre, open-label, randomised controlled trial. The Lancet [Internet]. 2024 Apr 8 [cited 2024 Apr 8];0(0). Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(24)00413-6/fulltext




No comments yet!