03 Nov 2024
Comparison of noncompliant balloon with drug-coating balloon angioplasties for side branch after provisional stenting for patients with true coronary bifurcation lesions: The prospective, multicenter, randomized DCB-BIF Trial
Reported from TCT 2024
Aaysha Cader provides her take on the DCB-BIF Trial presented by Shao-Liang Chen at TCT 2024 in Washington.
Why this study - the rationale/objective?
A provisional stenting strategy for coronary bifurcation lesions can frequently lead to side branch (SB) compromise, often requiring SB stenting and leading to suboptimal results. Drug-coated balloons (DCB) angioplasty could be a potential strategy for the compromised side branch, however, its benefit in coronary bifurcations is unclear. The DCB-BIF trial sought to investigate whether in patients with simple, true coronary bifurcation lesions, a DCB, as compared with a non-compliant balloon (NCB) angioplasty for the pinched SB improved outcomes (1, 2).
How was it executed - the methodology
The DCB BIF trial was an international multicentre randomised controlled trial that enrolled 784 patients with simple, true coronary bifurcation lesions in 22 centres in China, Indonesia, Italy, and Korea (1, 2). Patient presentations included silent ischemia, stable or unstable angina, or acute myocardial infarction (MI) >7 days.
The bifurcation lesions included were true ( i.e. Medina 1,1,1; 0,1,1; or 1,0,1) and simple (i.e. SB lesion length <10 mm as per DEFINITION criteria)(3). Provisional stenting was done as per European Bifurcation Club (EBC) recommendations and SB predilatation was not recommended. Additionally, target lesions were required to have a reference vessel diameter of ≥ 2.5 mm for both MV and SB, baseline diameter stenosis of ≥50%, and an ostial SB diameter stenosis of ≥70% following MV stenting and proximal optimisation technique (POT).
Participants were randomised following MV stenting 1:1 to receive either a DCB (paclitaxel-eluting) or NCB to treat the pinched SB. In the DCB arm, SB rewiring, NCB predilatation and DCB (sized 0.8-1:1) angioplasty were done, followed by kissing balloon inflation (KBI) and final POT. NCB arm had a similar protocol, excluding the DCB angioplasty step.
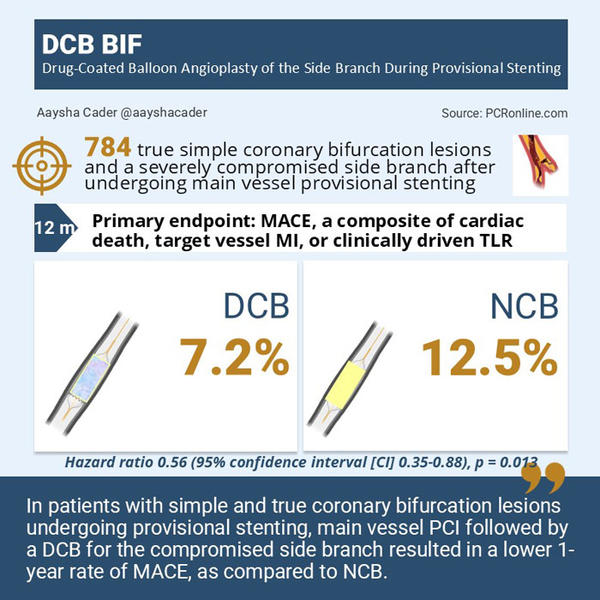
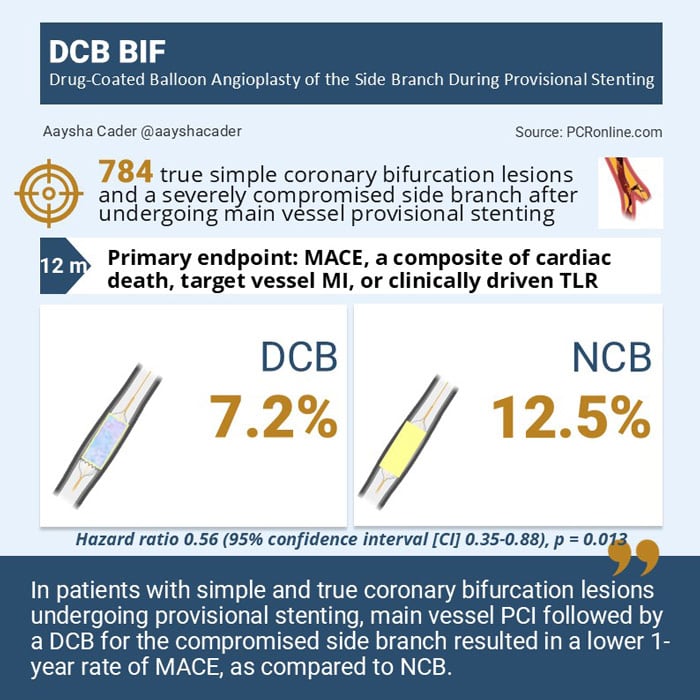
The primary endpoint was major adverse cardiac events (MACE), a composite of cardiac death, target vessel myocardial infarction, (TVMI) or clinically driven target-lesion revascularization (TLR) at the 1-year.
What is the main result?
The median patient age was 65 years and 76.7% were men. 76.1% of patients had Medina 1,1,1 bifurcation lesions. The majority of bifurcation lesions were in the left anterior descending artery 67.9%, and 15.2% had distal left main bifurcation lesions. Intravascular ultrasound (IVUS) was used in only 22.6% of cases and optical coherence tomography (OCT) in 4.7%. All patients in the DCB arm received SB preparation with an NCB after MV stenting. Overall, KBI was performed in 96.9% of patients, and re-POT was done in 83.9%.
Crossover from a 1-stent to 2-stent strategy occurred in 3.3% vs 3.8% in the NCB and DCB arms respectively (p=0.69).
The primary endpoint occurred in 7.2% in the DCB arm vs.12.5% in the NCB arm, corresponding to a 44% reduction in MACE with DCB ( hazard ratio (HR) 0.56 (95% confidence interval [CI] 0.35-0.88), p = 0.013). There was no interaction between subgroups.
Among key secondary endpoints, the risk of spontaneous MI was higher in the NCB group compared to the DCB group (3.6% vs 1.0%; HR: 0.27; 95% CI: 0.09-0.81; P=0.029), leading to a higher rate of target vessel myocardial infarction (TVMI) in the NCB group (10.9% vs 5.6%; HR: 0.50; 95% CI: 0.30-0.84; P=0.009).
No significant differences were seen in secondary endpoints of cardiac death, TLF without procedural MI, clinically-driven TLR and stent thrombosis (ST). Two definite STs were observed in the DCB arm.
Critical reading and the relevance for clinical practice
DCB BIF is the first powered, randomized trial to demonstrate superiority of a DCB over NCB in a compromised SB of simple true bifurcation lesions following provisional MV stenting.
The reduction in MACE with DCB was primarily driven by fewer TVMIs, more notably spontaneous MIs, although TLR was no different between the arms.
Furthermore, while most MIs occurred early, TLR occurred relatively later, possibly indicating that these MIs were not clinically significant to warrant intervention and probably detected by biomarker rise, given that serial troponins were protocol-mandated up to the first 48 hours of PCI. There were also two definite stent thrombosis events in the DCB arm, which had been attributed to chance.
The authors postulated that the longer duration of inflation at lower pressure may be one reason for reduced TVMI by DCB. That being said, only paclitaxel-eluting balloons were used in the trial, and therefore results may not be applicable to sirolimus-eluting balloons, given different mechanisms of DCBs.
From a technical standpoint, by using NCB for KBI after DCB, this study supports the routine use of NCB inflation after DCB in the SB, at low pressure to facilitate drug penetration into the vascular wall. The use of intravascular imaging was rather low overall, and further studies regarding the use of imaging to guide DCB angioplasty remain an area of further investigation.
Finally, the results of this study are only applicable to simple true bifurcation lesions, as complex lesions by DEFINITION criteria, severe calcific disease requiring rotational atherectomy, restenotic lesions, and acute MI <7 days were excluded.
References:
- Gao X, Tian N, Kan J, et al. Drug-Coated Balloon Angioplasty of the Side Branch During Provisional Stenting: The Multicenter Randomized DCB-BIF Trial. J Am Coll Cardiol 2024;Oct 28:[Epub ahead of print]
- Gao XF, Ge Z, Kan J, Kong XQ, Wang Y, Qiu CG, Tresukosol D, He YQ, Wu Q, Li JF, Yuan HT, Shen C, Chen X, Munawar M, Hanif B, Santoso T, Shin ES, Sheiban I, Ye F, Zhang JJ, Chen SL; DCB-BIF investigators. Rationale and design for comparison of non-compliant balloon with drug-coating balloon angioplasty for side branch after provisional stenting for patients with true coronary bifurcation lesions: a prospective, multicentre and randomised DCB-BIF trial. BMJ Open. 2022 Mar 11;12(3):e052788. doi: 10.1136/bmjopen-2021-052788. PMID: 35277400; PMCID: PMC8919455.
- Chen SL, Sheiban I, Xu B, et al. Impact of the complexity of bifurcation lesions treated with drug-eluting stents: the DEFINITION study (Definitions and Impact of Complex Bifurcation Lesions on Clinical Outcomes After Percutaneous Coronary Intervention Using Drug-Eluting Stents). JACC Cardiovasc Interv. 2014;7(11):1266–1276







2 comments
The results of the trial emphasise that the current concept of re-crossing after DES implantation in the main branch is not a good idea. The rate of periprocedural myocardial infarction is unacceptable. Basically, it is more or less irrelevant how the side branch is treated. DCBs can dramatically simplify bifurcation treatment. However, the concept here is to first perform lesion preparation in both branches one after the other, followed by DCB in the SB and DCB in the MB. No further action required. The EBC DCB study will compare exactly this concept with provisional T stenting. 750 patients will be randomized. Lets shape the future of interventional cardiology with innovative trials. The trial presented here does not have the potential to do so.
thanks for the comment. I totally agree that systematic re-crossing and dilatation is not a good idea. let's wait for the EBC DCB study: could you explain us a little bit more about the design and the two comparator arms?