TAVI and FFR-guided PCI vs. SAVR and CABG in AS with complex or multivessel CAD: the TCW Trial
Selected in The Lancet by N. Ryan
The TCW trial found that FFR-guided PCI with TAVI offers favorable one-year outcomes, including lower rates of all-cause mortality, MI, disabling stroke, revascularisation, valve reintervention, and severe bleeding, compared to CABG with SAVR.
References
Authors
Elvin Kedhi, Renicus S Hermanides, Jan-Henk E Dambrink, Sandeep K Singh, Jurriën M Ten Berg, DirkJan van Ginkel, Martin Hudec, Giovanni Amoroso, Ignacio J Amat-Santos, Martin Andreas, Rui Campante Teles, Guillaume Bonnet, Eric Van Belle, Lenard Conradi, Leen van Garsse, Wojtek Wojakowski, Vassilis Voudris, Jerzy Sacha, Pavel Cervinka, Erik Lipsic, Samer Somi, Luis Nombela-Franco, Sonja Postma, Kerstin Piayda, Giuseppe De Luca, Evelien Kolkman, Krzysztof P Malinowski, Thomas Modine, on behalf of the TCW study group
Reference
DOI: 10.1016/S0140-6736(24)02100-7
Published
December 04, 2024
Link
Read the abstractReviewer
Latest contributions
Balloon lithotripsy added to conventional preparation before stent implantation in severely calcified coronary lesions Impact of pullback pressure gradient on clinical outcomes after percutaneous coronary interventions Validation of intravascular ultrasound-defined optimal stent expansion criteria for favorable 1-year clinical outcomesMy Comment
TCW compared FFR-guided PCI and TAVI with the Evolut R/Pro TAVI valve to CABG and SAVR with a bioprosthetic valve in patients with severe aortic stenosis and complex coronary artery disease with equipoise for either treatment.
Designed by Nicola Ryan - Source: PCRonline
Why this study – the rationale/objective?
Concomitant coronary artery disease is common in patients with severe aortic stenosis, with current guidelines recommending CABG and SAVR. Combined CABG and SAVR has an increased mortality risk compared to the individual procedures. TAVI has been demonstrated to be non-inferior or superior to SAVR in terms of short and intermediate term outcomes in low and intermediate risk patients. Similarly, in patients with a low SYNTAX score, PCI is equivalent to CABG.
How was it executed – the methodology?
Patients aged ≥ 70 years with severe symptomatic aortic stenosis with concomitant multivessel or complex coronary artery disease; ≥ 50% stenosis in an artery > 2 mm or a single LAD lesion > 20 mm involving a bifurcation with a diagonal, were eligible for inclusion if the on-site Heart Team deemed them suitable for percutaneous intervention (FFR guided PCI and TAVI) and Conventional Surgery (CABG and SAVR).
Patients randomised to percutaneous intervention underwent PCI to all lesions with an FFR ≤ 0.80 followed by TAVI with a CoreValve Evolut R or Pro with commissural alignment. Use of arterial grafts was strongly recommended in the CABG group with FFR use at the surgeons discretion, bioprosthetic surgical valves were used. A non-inferiority design was used with a non-inferiority margin of 15 %.
- The primary endpoint was a POCE all-cause mortality, myocardial infarction, disabling stroke, unscheduled clinically driven target vessel revascularisation, valve reintervention and life threatening or disabling bleeding at one year
- The secondary endpoints were:
- MACE: cardiovascular mortality, all stroke, myocardial infarction, unscheduled coronary events or valve reintervention at one year
- A composite of all-mortality and stroke at one year
What is the main result?
Overall, from May 2018 to June 2023, 172 patients were enrolled, 91 randomised to the percutaneous intervention arm, and 81 to conventional surgery.
In the percutaneous arm, 89 received PCI & TAVI, 1 CABG & SAVR and 1 PCI alone; in the conventional surgery arm, 64 received CABG & SAVR, 7 PCI & TAVI, 1 PCI alone and 1 TAVI alone. The majority (69 %) of patients were male, with a mean age of 76.5 years. The patients had a low surgical risk with a median STS and EuroSCORE II of 2.4. The majority (72 %) of patients had a low SYNTAX Score.
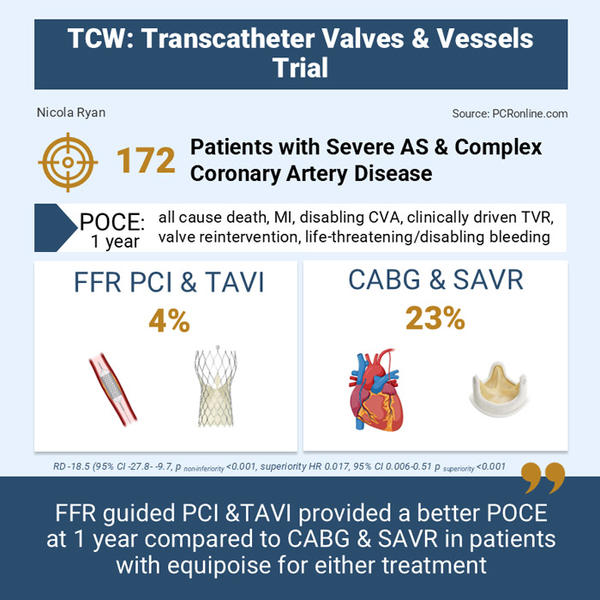
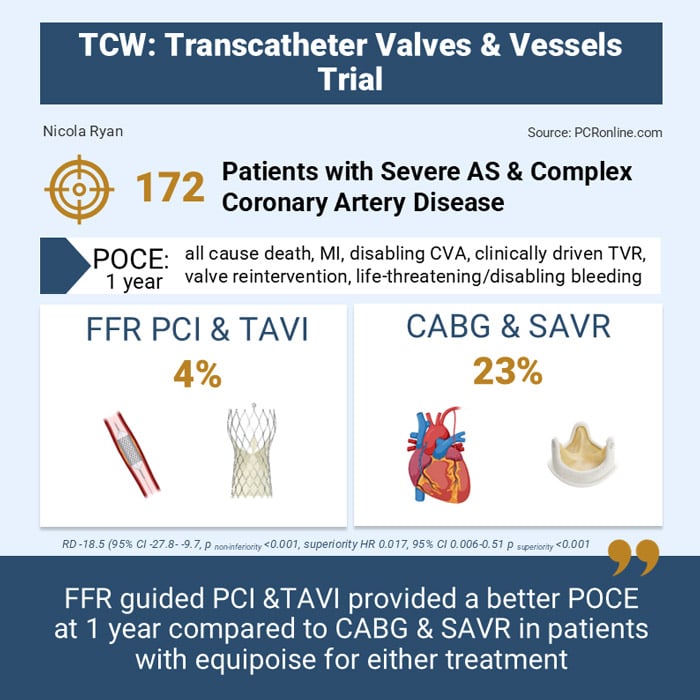
- POCE at one year was 4 % in the percutaneous intervention group vs 23 % in the conventional surgery group (risk difference -18.5 %, 95 %CI -27.8 - -9.7, pnon-inferiority < 0.001)
- Given non-inferiority was met, superiority was tested which showed that percutaneous intervention was superior to conventional surgery in terms of POCE at one year (HR 0.17, 95 % CI 0.06-0.51, psuperiority < 0.001)
- MACE was lower in the percutaneous intervention group (3 % vs 14 %, HR 0.23, 95 % CI 0.6-0.83, p = 0.014) at one year.
- The composite of all-cause death and CVA was lower in the percutaneous intervention group (1 % vs 12 %, HR 0.8, 95 % CI 0.01-0.66, p = 0.0027) at one year.
Critical reading and the relevance for clinical practice
The TCW trial demonstrated that, in patients with severe aortic stenosis and multivessel or complex coronary artery disease, percutaneous intervention with FFR-guided PCI and TAVI is superior to conventional surgery with CABG and SAVR.
The trial was designed assuming a POCE rate of 30 % in the CABG arm, with a non-inferiority margin of 15 % and a planned enrolment of 328 patients. The trial was stopped early due to an important difference in event rates between groups seen on the safety data analysis. There was a relatively high cross over rate in the conventional surgery arm; however, the difference in POCE was also seen when the population was analysed as treated and per protocol.
The majority of the difference between groups was due to increased all-cause mortality (10 % vs 0 %, p = 0.0025) and life-threatening bleeding (12 % vs 2 %, p = 0.01) in the surgery group. Of the seven deaths in the trial, all occurred in the conventional surgery group, with six cardiovascular deaths and five procedural related. Adding CABG to SAVR alone increases the complexity and duration of the procedure; however, it is currently recommended that coronary lesions requiring revascularisation are treated concomitantly with SAVR in patients undergoing conventional surgery. The role of PCI in patients undergoing TAVI is not well defined, however, where PCI alone is indication functionally complete revascularisation gives better outcomes. The role of coronary intervention in patients having undergone successful TAVI is being investigated in the COMPLETE TAVR trial.
This trial included only one brand of self-expanding valve (SEV) therefore generalisability to other brands of SEV and balloon-expanding valves cannot be assumed. Four surgical bioprosthetic valves were utilised during the trial, which introduces heterogeneity to the group, whilst the majority of valves were Perimount Magna Ease. Trifeca valves were implanted in 10 patients, which is important to bear in mind during longer term follow-up.
The role of FFR in patients with severe AS has been a matter of debate, while FFR may underestimate stenosis severity in patients with AS; this is only of clinical consequence in borderline lesions, therefore as with all situations, the clinical context and lesion location should be considered when FFR measurements are borderline negative in patients with severe AS. The majority of patients in this trial had low SYNTAX scores, therefore the applicability to more complex coronary disease is unknown. By nature of the trial design, patients considered unsuitable for either modality were excluded; therefore the results are only applicable in patients where the Heart Team deems both a percutaneous and surgical approach feasible.
The TCW trial has shown that FFR-guided PCI and TAVI gives favourable outcomes in terms of the patient orientated composite outcome of all-cause mortality, myocardial infarction, disabling stroke, unscheduled clinically driven target vessel revascularisation, valve reintervention and life threatening or disabling bleeding compared to CABG and SAVR at one year.
Overall, the numbers enrolled in the trial were small and larger randomised trials with both self-expanding and balloon expanding valves are required.







No comments yet!