Rivaroxaban monotherapy in patients with atrial fibrillation after coronary stenting: insights from the AFIRE trial
Selected in JACC: Cardiovascular Interventions by N. Ryan
In this prespecified subgroup analysis of the AFIRE trial, the authors report the outcomes of rivaroxaban monotherapy compared to rivaroxaban and an antiplatelet agent in patients with atrial fibrillation who underwent coronary stenting > 1 year prior to enrolment.
References
Authors
Tetsuya Matoba, Satoshi Yasuda, Koichi Kaikita, Masaharu Akao, Junya Ako, Masato Nakamura, Katsumi Miyauchi, Nobuhisa Hagiwara, Kazuo Kimura, Atsushi Hirayama, Kunihiko Matsui, Hisao Ogawa, and AFIRE Investigators
Reference
J Am Coll Cardiol Intv. 2021 Nov, 14 (21) 2330–2340
Published
November 2021
Link
Read the abstract
Reviewer
Latest contributions
Balloon lithotripsy added to conventional preparation before stent implantation in severely calcified coronary lesions Impact of pullback pressure gradient on clinical outcomes after percutaneous coronary interventions Validation of intravascular ultrasound-defined optimal stent expansion criteria for favorable 1-year clinical outcomesMy Comment
Why this study – the rationale/objective?
In patients with concomitant atrial fibrillation and coronary artery disease undergoing coronary stenting, the management of anticoagulation and antiplatelet therapy remains a dilemma.
Current guidelines recommend anticoagulant monotherapy at one year in most patients. Despite this, discontinuation of all antiplatelet agents and the potential risk of stent thrombosis remains a concern.
The AFIRE trial enrolled patients with atrial fibrillation and stable coronary artery disease.
The trial was stopped early due to increased mortality in the combination therapy group: it showed that rivaroxaban monotherapy was non-inferior to combination of rivaroxaban and an antiplatelet agent.
This pre-specified subgroup analysis examines the outcomes of patients who had undergone coronary stenting prior to enrolment in the trial.
How was it executed? - the methodology
Patients with atrial fibrillation (CHADS2 ≥ 1) and stable CAD were randomised to monotherapy with rivaroxaban (CrCl 15-49ml/min – 10mg, >50ml/min – 15mg) or rivaroxaban with aspirin or a P2Y12 inhibitor (at the discretion of the treating physician). The outcomes were analysed by time from stenting to enrolment in the trial and type of stent implanted (BMS, 1st or 2nd generation DES). Exclusion criteria included a history of stent thrombosis.
- The primary efficacy endpoint was a composite of all-cause death, MI, stroke, systemic embolism, MI, and unstable angina.
- The primary safety endpoint was major bleeding according to the ISTH criteria.
- Secondary endpoints included ischaemic events a composite of cardiovascular death, ischaemic stroke, MI, unstable angina requiring revascularisation or stent thrombosis, and NACE a composite of ischaemic endpoints and major bleeding.
What is the main result?
Overall, 1,444 patients who had undergone coronary stenting prior to enrolment were included in this analysis, with a median follow-up of 23.6 months (17.2-30.8).
723 were randomised to rivaroxaban monotherapy and 721 to combination therapy ( two thirds treated with aspirin and one third with a P2Y12 inhibitor. The majority of patients were male (80.7 %) with 52.2 % ≥ 75years, diabetes was present in 43.3 %. Over half of patients had a single stented segment (57.8 %) with a mean time from stenting of 47.6 months (20.9-91.4), 71 % of participants underwent stenting more than two years prior to enrolment.
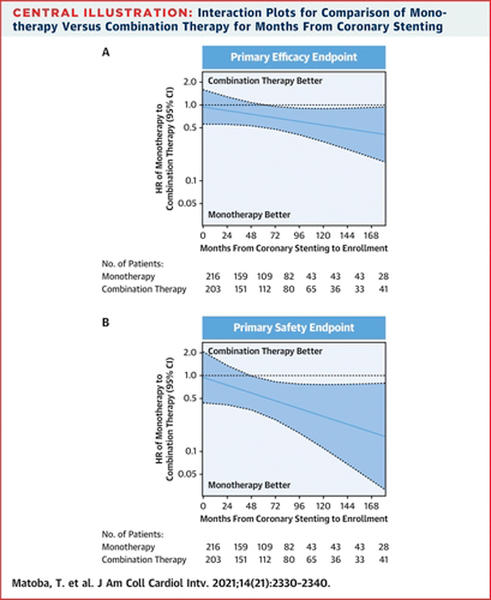
- The primary efficacy endpoint occurred in 58/723 patients in the monotherapy arm and 82/821 in the combination therapy arm. Incidence rates of 4.13 % vs 5.84 % per patient-year (HR 0.70, 95 % CI 0.50-0.98, p = 0.036).
- The primary safety endpoint had incidence rates of 1.69 % vs 3.06 % per patient-year in the monotherapy and combination therapy arms respectively (HR 0.55, 95 % CI 0.33-0.92, p = 0.019).
- For the efficacy endpoint, there was a significant interaction between age ≥ 75 years and the benefit of monotherapy (p for interaction = 0.026).
- There were no differences in ischaemic endpoints between groups 60/723 vs 73/721 in the monotherapy and combination therapy arms respectively. Incidence rates 4.33 % vs 5.32 % per patient-year (HR 0.82, 95 % CI 0.58-1.15, p = 0.240)
- Incidence rate of NACE were 6.28 % vs 9.21 % per patient-year in the monotherapy (87/723) and combination (122/721) arms, (HR 0.69, 95 % CI 0.53-0.91, p = 0.009).
Source: JACC: Cardiovascular Interventions
Critical reading and the relevance for clinical practice
The results of this subgroup analysis show that rivaroxaban monotherapy is non-inferior to combination therapy with rivaroxaban and an antiplatelet agent in patients with atrial fibrillation and stable coronary artery disease more than one year post coronary stenting.
Importantly, there were no significant differences in ischaemic endpoints amongst the monotherapy and combination therapy groups. There were numerically higher coronary events (MI, PCI, CABG) in the monotherapy are which at subgroup analysis was particularly apparent in the 15 mg rivaroxaban arm. Whilst 15 mg is not a standard dose of rivaroxaban in Europe, it is the standard dose prescribed in Japan for patients with a CrCl >50ml/min. Further research is required to understand whether this translates into an increased thrombotic risk in patients treated with rivaroxaban monotherapy, particularly at the more standard European dose of 20 mg. Rivaroxaban monotherapy reduced bleeding endpoints in the population as a whole, with no important differences amongst subgroups.
This sub-analysis provides important data to support the current recommendations for discontinuation of anti-platelet therapy in most patients at one year, however, there is a number of important limitations that must be borne in mind.
Firstly, by nature of the trial design, patients who physicians felt required combination therapy will not have been enrolled in this study. While screening data is not available to understand this population given that over half of the enrolled population had single vessel stenting it is likely that patients more at risk for ischaemic events, i.e. multi-vessel, long stented segments, bifurcation stenting were less likely to be included. Whilst the authors did not find any differences in outcomes based on the stent type implanted, it would be of interest to understand the outcomes based on complexity of stenting.
Secondly, whilst current guidelines recommend monotherapy from 12 months in the majority of patients in this subgroup analysis were enrolled over 24 months, since their initial stenting procedure, this additional time may allow more complete endothelialisation and thus reduced the risk of thrombotic events, of note there was no stent thrombosis reported in the AFIRE trial. Previous stent thrombosis was an important exclusion criterion in this trial, therefore these results cannot be extrapolated to this population.
Historically, the focus within the PCI community has been on the ischaemic risk post PCI, however, it is now understood that bleeding carries an important morbidity and mortality therefore minimising bleeding without increasing ischaemic risk is a careful balance.
Overall this study adds strength to the recommendation that antiplatelet therapy can be safely discontinued in carefully selected patients at 12 months post stenting. Further research is required to understand the risks and benefits of anticoagulation alone in patients undergoing complex PCI after one year.




No comments yet!