Recurrent events analysis of MASTER DAPT: total ischemic and bleeding events after abbreviated vs prolonged DAPT in HBR patient
Selected in JACC by N. Ryan
This exploratory analysis from the MASTER DAPT trial evaluates the effect of abbreviated versus prolonged DAPT on total ischaemic and bleeding events in high-bleeding-risk patients from the MASTER DAPT trial.
References
Authors
Dario Bongiovanni, Antonio Landi, Enrico Frigoli, Dik Heg, Konstantina Chalkou, Jozef Bartunek, Laurent Delorme, Willem Dewilde, David Hildick-Smith, Gregor Leibundgut, Sergio Leonardi, Maciej Lesiak, Petr Kala, Sasko Kedev, Marco Roffi, Goran Stankovic, Pim A.L. Tonino, Vasil Velchev, Pascal Vranckx, Stephan Windecker, Pieter C. Smits, and Marco Valgimigli
Reference
Epublished DOI: 10.1016/j.jacc.2025.05.010
Published
May 22, 2025
Link
Read the abstractReviewer
Latest contributions
Balloon lithotripsy added to conventional preparation before stent implantation in severely calcified coronary lesions Impact of pullback pressure gradient on clinical outcomes after percutaneous coronary interventions Validation of intravascular ultrasound-defined optimal stent expansion criteria for favorable 1-year clinical outcomesMy Comment
Designed by: Nicola Ryan
Source: PCRonline.com
Why this study – the rationale/objective?
Dual antiplatelet therapy aims to reduce ischaemic risk after ACS and PCI, but comes with a concomitant increased risk of bleeding.
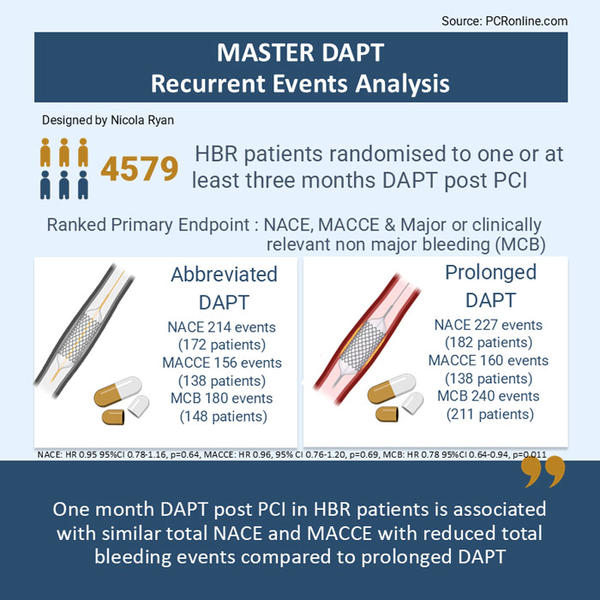
The MASTER DAPT trial enrolled patients at high bleeding risk undergoing PCI with a biodegradable polymer coated stent (Ultimaster or Ultimaster TANSEI). Following 30 days DAPT treatment with no clinical events, patients were randomised to single antiplatelet therapy (abbreviated DAPT) or standard dual antiplatelet therapy (prolonged DAPT). Patients were followed for 335 days with three co-primary endpoints (hierarchical testing). Non-inferiority was met for net adverse clinical endpoints (NACE 7.5 % vs 7.7 %, p < 0.001) and major adverse cardiac and cerebral events (MACCE 6.1 % vs 5.9 % , p < 0.001) and superiority for major or clinically relevant non-major bleeding (MCB: 6.5 % vs. 9.4 %, p < 0.001)1.
The design of the MASTER DAPT trial, similar to many censors the patient following the occurrence of a component of the endpoint, this underestimates the total burden of disease in the population and does not account for recurrent events which have important clinical implications.
In this analysis, the authors examined the effects of abbreviated DAPT on the total occurrence of any components of the primary endpoint, including recurrent events.
How was it executed - the methodology
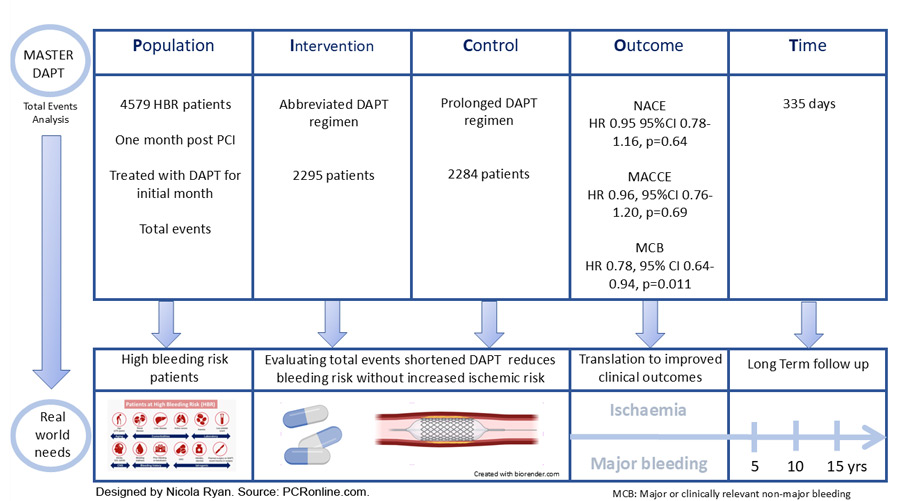
High-bleeding-risk patients undergoing PCI with a biodegradable polymer-coated stent for acute or chronic coronary syndrome were eligible for inclusion. Patients underwent 1:1 randomisation to abbreviated or prolonged antiplatelet therapy 30-44 days after the initial procedure, provided no ischaemic events occurred. Patients receiving OACs assigned to abbreviated DAPT continued SAPT and OAC for up to six months post the index procedure, whilst those randomised to prolonged DAPT continued DAPT for three months post index procedure, then continued on SAPT and OAC.
- The primary outcome was three ranked measures NACE – a composite of all cause death, MI stroke or major bleeding (BARC 3 or 5), MACCE – a composite of all cause death, MI or stroke and MCB – a composite of BARC type 2, 3 or 5 bleeding.
- Secondary outcomes were the individual components of the primary endpoint.
- The impact of clinical events on antithrombotic therapy was assessed: Upgraded: enhancement of antithrombotic therapy; Unchanged: no relevant changes; Downgraded: interruption of antithrombotic agent or change to a less potent drug.
The effects of abbreviated versus prolonged DAPT on outcomes in this analysis was evaluated using an intention-to-treat analysis to capture all clinical events and reflect real-world clinical outcomes.
What is the main result?
Designed by: Nicola Ryan
Source: PCRonline.com
Of the 4,579 patients included in this analysis, 96.3 % experienced no NACE, 6.25 % (283 patients) experienced one NACE, and 1.6 % (71 patients) experienced two or more NACE. One MACCE occurred in 5.2 % (239 patients) and ≥ 2 MACCE in 0.8 % (37 patients), 6.9 % (317 patients) had one MCB and 0.9 % (42 patients) had ≥ 2 MCB. Patients who had recurrent MACCE more commonly presented with NSTEMI, whilst OAC use was more common in patients with recurrent MCBs.
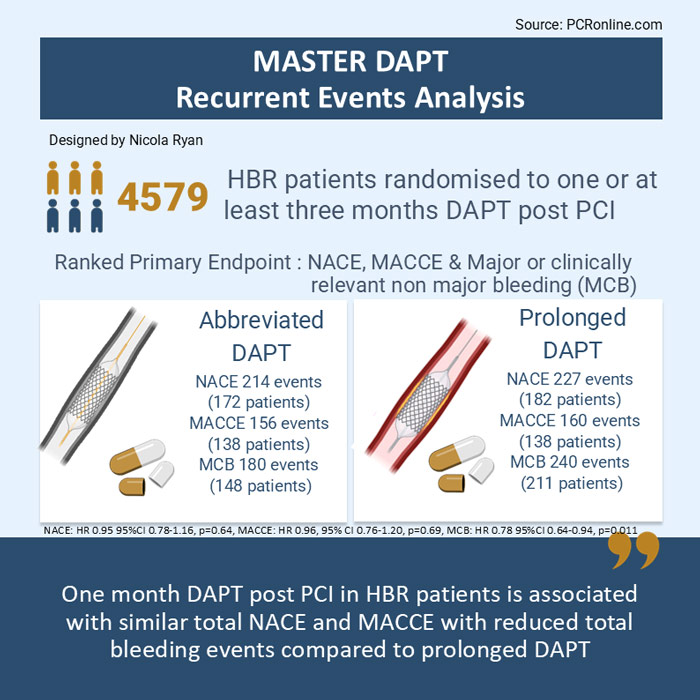
- Total NACE was similar between groups, abbreviated DAPT group 214 vs. 227 prolonged DAPT group (HR 0.95, 95 % CI 0.78-1.15, p = 0.64)
- The incidence rate ratio for recurrent NACE did not differ between groups (IRR 0.93, 95 % CI 0.61-1.41, p = 0.72)
- Total MACCE was similar between groups, abbreviated DAPT 156 vs. prolonged DAPT 160 (HR 0.96, 95 % CI 0.76-1.20, p = 0.69)
- There was no difference in recurrent MACCE between groups, 18 abbreviated DAPT vs 22 prolonged DAPT (IRR 0.82, 95 % CI 0.53-1.51, p = 0.51)
- Total MCB was lower in the abbreviated DAPT group 180 vs prolonged DAPT 240 (HR 0.78, 95 % CI 0.64-0.94, p = 0.011)
- There were no differences in recurrent MCB (IRR 1.1, 95 % CI 0.66-1.82, p = 0.72)
- 71 out of a total of 316 MACCE events (22.5 %) occurred after a BARC bleeding with no significant difference between abbreviated and prolonged DAPT groups (IRR 0.82, 95 % CI 0.51-1.30, p = 0.39)
- 26 out of a total of 628 BARC bleeding events (4.1 %) occurred after a MACCE event with no significant difference between abbreviated and prolonged DAPT (IRR 1.36, 95 % CI 0.62-3.02, p = 0.45)
Critical reading and the relevance for clinical practice:
The results of this exploratory analysis show that abbreviated DAPT in HBR patients, who are event-free 30 days post PCI with a biodegradable polymer-coated stent, is associated with a similar rate of total NACE and MACCE, but lower total bleeding events compared to prolonged DAPT. Recurrent events are not uncommon accounting for 19.7 % of total NACE, 12.6 % of MACCE and 14.5 % of MCB. Importantly, a fifth of all MACCE occurred after a bleeding event, whilst only 4 % of bleeding events occurred after a MACCE, therefore bleeding prevention appears to be a key consideration to reduce risk in this population.
Historically there has been significant concern with regard to ischaemic events post PCI, however, even when taking recurrent events into account, abbreviated DAPT did not have an ischaemic penalty. Whilst there was no difference in overall MACCE, abbreviated DAPT was associated with fewer total CVA (HR 0.51, 95 % CI 0.28-0.91) and total stroke (HR 0.49, 95 % CI 0.25-0.98) compared to prolonged DAPT. Following an MI antithrombotic therapy was more commonly ungraded in the abbreviated DAPT group. Despite no overall differences in MI, the time to recurrent MI was three times longer in the abbreviated DAPT group supporting the safety of an abbreviated DAPT strategy in this population.
Patients treated with abbreviated DAPT had lower total BARC 2 and total and recurrent BARC 1 bleeding compared to the prolonged DAPT group. Whilst total BARC 3 or 5 bleeding was numerically lower in the abbreviated DAPT group, it did not reach statistical significance. In patients with bleeding events, antithrombotic therapy was downgraded with a similar frequency after a BARC 2 bleed in both groups however not after a BARC 1 bleed. This downgrading of DAPT may explain the lack of difference in recurrent MCB’s between groups and reflects real world clinical practice.
Importantly, three quarters of patients in the MASTER DAPT trial continued with P2Y12 monotherapy as opposed to aspirin monotherapy, however, the optimal single antiplatelet strategy post PCI remains an open topic. An analysis of the TWILIGHT trial2, where patients with bleeding and ischaemic risk factors were randomised to withdrawal of aspirin at three months or continued DAPT, showed similar rates of recurrent MACCE but lower recurrent bleeding events compared to the MASTER DAPT population. The exclusion of patients requiring OAC therapy from the TWILIGHT study likely explains some of this difference in recurrent bleeding as patients treated with OACs in MASTER DAPT more commonly had recurrent bleeding events. Furthermore, both ischaemic and bleeding risk were inclusion criteria for the TWILIGHT trial whereas MASTER DAPT focused on a HBR population.
There is a number of limitations to the study, none the least the open label design, whilst this may introduce bias, it also reflects real-life clinical practice. The patient population included were a HBR population free from ischaemic events at one month, therefore the results cannot be generalised to the non-HBR population or those with an ischaemic event in the first month. Furthermore, all patients within the MASTER DAPT trial were treated with a biodegradable polymer-coated stent, however, shortened DAPT has been evaluated with other stent platforms total and recurrent event data has not been reported.
This data highlights the importance of both recurrent events and the full spectrum of clinical events, which are not typically captured in clinical trial results, due to the nature of trial design, but are commonly encountered in real-life clinical practice. Almost a fifth of the participants experienced a second NACE, with ischaemic events more commonly occurring after bleeding events. Though the underlying aetiology of this increased ischaemic risk post bleeding could not be established in this dataset, there are several potential mechanisms which warrant further investigation. Abbreviated DAPT appears a reasonable strategy in a HBR population similar to that enrolled in the MASTER DAPT trial. Of note, this study only includes patients who were event free at 30 days therefore, this is a strategy that can only be applied following clinical review at one month. Finally, DAPT duration is a dynamic decision and requires continuous evaluation and re-evaluation dependent on the clinical evolution of the patient.
References
- Valgimigli Marco, Frigoli Enrico, Heg Dik, Tijssen Jan, Jüni Peter, Vranckx Pascal, et al. Dual Antiplatelet Therapy after PCI in Patients at High Bleeding Risk. New England Journal of Medicine. 2021 Oct 27;385(18):1643–55.
- Baber U, Cao D, Collier T, Sartori S, Dangas G, Angiolillo DJ, et al. Impact of ticagrelor with or without aspirin on total and recurrent bleeding and ischaemic events after percutaneous coronary intervention: a sub-study of the TWILIGHT trial. European Heart Journal - Cardiovascular Pharmacotherapy. 2025 Jan 1;11(1):66–74.




No comments yet!