Coronary lithotripsy for the treatment of underexpanded stents: the international multicentre CRUNCH registry
Selected in EuroIntervention Journal by A. N. Calik , I. Polat Canbolat
CRUNCH registry aimed to investigate the safety and efficacy of intravascular lithotripsy in the setting of resistant stent under expansion due to underlying calcific plaques.
References
Authors
Maria Natalia Tovar Forero, Gennaro Sardella, Nicolò Salvi, Bernardo Cortese, Gaetano di Palma, Nikos Werner, Adem Aksoy, Javier Escaned, Carlos H. Salazar, Nieves Gonzalo, Fabrizio Ugo, Chiara Cavallino, Tej N. Sheth, Isabella Kardys, Nicolas M. Van Mieghem, Joost Daemen
Reference
10.4244/EIJ-D-21-00545
Published
23 March 2022
Link
Read the abstract
Reviewers
Our Comment
Why this study – the rationale/objective?
Appropriate plaque modification prior to stent implantation is essential to allow adequate device deployment.
Stent under expansion (SU) is strongly associated with stent restenosis and thrombosis.
The intravascular lithotripsy (IVL) system (Shockwave Medical Inc.) is a new coronary calcium modifying tool on the market, consisting of a semi-compliant balloon with integrated lithotripsy electrodes and an electrical pulse generator that produces unfocused circumferential mechanical energy to crack calcified plaques.
CRUNCH registry aimed to investigate the safety and efficacy of IVL in the setting of resistant SU due to underlying calcific plaques.
How was it executed? - the methodology
CRUNCH registry was an international multicenter registry including all patients who underwent IVL therapy to treat SU. The use of pre- and post- IVL dilatation with NC balloons, additional stenting, and the concomitant use of intracoronary imaging to guide the procedure were at the operator's discretion.
Reference stent diameter, maximum, mean, and minimum lumen diameters, and percentage diameter stenosis (% DS) were analyzed with CAAS 8.0 software for offline quantitive coronary analysis (QCA). Intracoronary imaging parameters; maximum, mean, and minimum lumen area (MaxLA, MeanLA, and MLA), and minimum stent area (MSA) were analyzed with QCU-CMS 4.69 software. Clinical, procedural, angiographical, and intracoronary imaging data were assessed in a centralized core lab.
The primary efficacy endpoint was device success, a composite of technical success (successful lesion crossing + successful delivery of intended number of IVL pulses + successful retrieval of the device), and residual % DS < 50 % as assessed by offline QCA analysis.
The primary safety endpoint was in-hospital major adverse cardiac events (MACE), defined as a composite of cardiac death, non-fatal myocardial infarction, and any repeat revascularization.
What is the main result?
A total of 70 patients were included in the study. The indication for PCI was acute coronary syndrome in 37 (52.8 %) patients. In 29 (41.4 %) patients, IVL was used as a bailout therapy to improve SU. Multiple stent layers were present in 15 (21.4 %) patients. Pre- and post-IVL dilatation with NC balloons, sized in a ratio ≥ 1:1 with respect to the nominal stent size, were used in 37 (69.8 %) and 41 (77.4 %) cases, respectively, and 15 (22.4 %) patients received additional stenting.
Offline paired QCA data analysis revealed a significant gain in lumen diameter from 1.49 ± 0.73 mm to 2.41 ± 0.67 mm (p < 0.001) with a significant reduction in % DS of 57.08 ± 26.85 % (p < 0.001).
Intracoronary imaging was used to guide the procedure in 44 (62.9 %) cases. Paired data analysis demonstrated a significant gain in MLA from 3.93 ± 2.41 mm2 to 5.88 ± 2.09 mm2 (p < 0.001), supported by a significant increase in MSA from 4.32 ± 3.20 mm2 to 6.45 ± 2.01 mm2 (p < 0.004).
In multivariable analysis, both bailout IVL therapy directly after stenting (β –0.49, 95 % CI: –0.52 to –0.10; p = 0.004) and ostial location of the target stented segment (β –0.39, 95 % CI: –0.59 to –0.07; p = 0.013) negatively impacted lumen diameter gain.
The median hospital stay was 2 (1-5) days, and no MACE was reported related to the device. Two patients died due to non-cardiac causes.
Central illustration. Coronary lithotripsy effect in stent underexpansion. Source: Eurointervention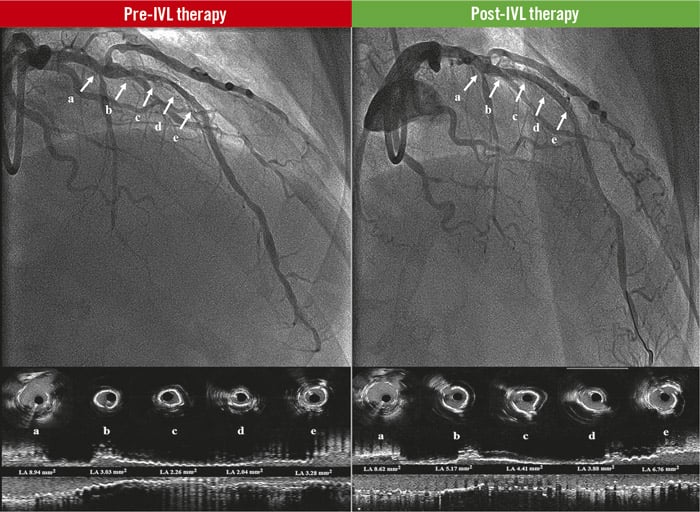
Pre-IVL therapy: baseline angiogram of the LAD and the corresponding coronary intravascular ultrasound cross-sections (a, b, c, d, e), and longitudinal view of the vessel. The white arrows show significant stent underexpansion with lumen obstruction.
Post-IVL therapy: final result angiograms after coronary lithotripsy therapy of the LAD and the corresponding coronary intravascular ultrasound cross sections (a, b, c, d, e), and longitudinal view of the vessel. The white arrows show significant improvement of stent expansion and lumen obstruction. IVL: intravascular coronary lithotripsy; LAD: left anterior descending coronary artery
Critical reading and the relevance for clinical practice?
In this international multicenter registry, IVL was found to be safe and effective for the treatment of resistant SU. The IVL system had a high device and technical success with no device-related procedural complications.
The previous DISRUPT CAD studies demonstrated the efficacy of lithotripsy in achieving a residual DS < 50 % in calcified native coronary arteries. The results of the current study extended these findings to the setting of prior stented coronary segments and showed that IVL therapy could reach that goal in 97 % of the cases, accounting for an overall device success of 92.3 %. It is important to note that residual diameter stenosis < 20 % was reached only in 53 % of the patients, which is the standard threshold to evaluate the effectiveness of coronary intervention devices for de novo lesions.
Although IVL for the treatment of stent under expansion remains “off-label”, the present study highlights that IVL therapy was an effective and safe strategy, especially when other devices have failed or entail an unacceptable, higher risk for complications.
Nonetheless, the results should be considered hypothesis-generating and require confirmation by larger-scale randomised studies evaluating the efficacy and safety of the IVL therapy versus other available devices in this specific context and its impact on the stent backbone/polymer and drug elution.
In short, the IVL device is a new tool in the calcium cracking market. It is easy to use and has high delivery success with low complication rates in patients with SU.







No comments yet!