06 Nov 2023
Guiding intervention for complex coronary lesions by optical coherence tomography or intravascular ultrasound
Selected in JACC by N. Ryan
The OCTIVUS trial was a prospective multicentre, open-label, randomised controlled trial comparing OCT guided PCI with IVUS guided PCI. In this pre-specified sub-study, the authors report the outcomes in patients with complex coronary lesions undergoing OCT- or IVUS-guided PCI.
References
Authors
Do-Yoon Kang, Jung-Min Ahn, Sung-Cheol Yun, Seung-Ho Hur, Yun-Kyeong Cho, Cheol Hyun Lee, Soon Jun Hong, Subin Lim, Sang-Wook Kim, Hoyoun Won, Jun-Hyok Oh, Jeong Cheon Choe, Young Joon Hong, Yong-Hoon Yoon, Hoyun Kim, Yeonwoo Choi, Jinho Lee, Young Won Yoon, Soo-Joong Kim, Jang-Ho Bae, Seung-Jung Park, Duk-Woo Park, and for the OCTIVUS Investigators
Reference
J Am Coll Cardiol . 2023 Oct 17:S0735-1097(23)07816-6. doi: 10.1016/j.jacc.2023.10.017. Online ahead of print.
Published
Available online 23 October 2023
Link
Read the abstractReviewer
Latest contributions
Balloon lithotripsy added to conventional preparation before stent implantation in severely calcified coronary lesions Impact of pullback pressure gradient on clinical outcomes after percutaneous coronary interventions Validation of intravascular ultrasound-defined optimal stent expansion criteria for favorable 1-year clinical outcomesMy Comment
The OCTIVUS trial was a prospective multicentre, open-label, randomised controlled trial comparing OCT-guided PCI with IVUS-guided PCI. In the main study, OCT-guided PCI was non-inferior to IVUS-guided PCI in terms of target vessel failure, the composite of cardiac death, target vessel MI or ischaemia drive target vessel revascularisation at one year1.
In this pre-specified sub-study, the authors report the outcomes in patients with complex coronary lesions undergoing OCT- or IVUS-guided PCI.
Why this study – the rationale/objective?
The use of intravascular imaging, particularly IVUS, has been shown to improve PCI outcomes compared to angiographic guidance alone. More recent studies have shown similar benefits with IVUS- and OCT- guided PCI, however, there are limited data directly comparing OCT with IVUS.
The OCTIVUS trial was designed to demonstrate the non-inferiority of OCT-guided PCI to IVUS and met its primary endpoint. Given that patients undergoing complex PCI have an increased risk of complications, they may be a subgroup which particularly benefits from intravascular imaging. It is therefore of interest to understand if there is a difference in outcomes based on imaging modality in this group; this pre-specified sub-study of the OCTIVUS trial aimed to address this question.
How was it executed – the methodology?
Patients undergoing clinically indicated PCI for chronic coronary syndrome or NSTEMI without anatomical features which would prevent delivery of an imaging catheter were eligible for inclusion. Patients were randomised in a 1:1 fashion to OCT-guided PCI or IVUS-guided PCI.
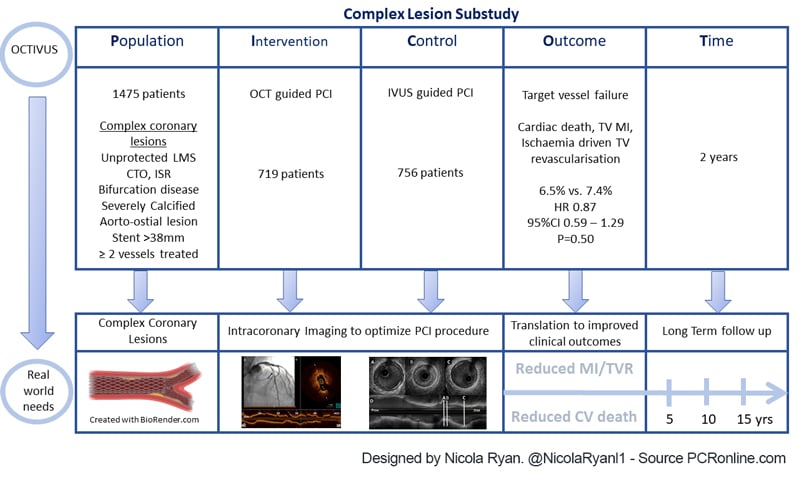
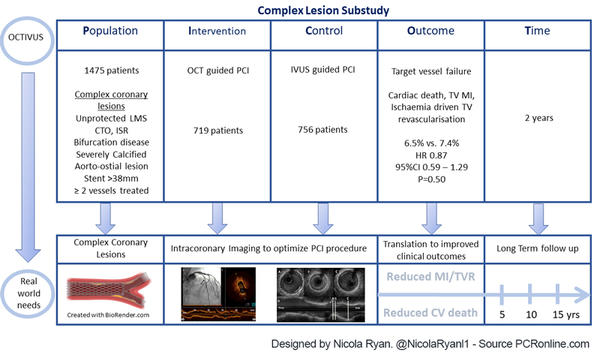
Complex PCI was defined as unprotected LMS, bifurcation disease, an aorto-ostial lesion, a chronic total occlusion, a severely calcified lesion, an in-stent restenotic lesion, long diffuse disease > 38 mm stent treated, or multivessel PCI involving at least two major epicardial coronary arteries. Imaging was used before, during and after PCI, with a final evaluation for PCI optimisation mandated.
Imaging criteria for optimisation included a distal lumen or external elastic membrane reference-based stent sizing strategy. Sufficient stent expansion was defined as ≥ 80 % of the mean reference lumen area, with no major stent malapposition or edge dissection.
- The primary endpoint was target vessel failure ; a composite cardiac death, target vessel related MI, and ischaemia driven target vessel revascularisation
- Key secondary endpoints included the individual components of the primary endpoint, target lesion failure (cardiac death, target vessel MI and ischaemia drive target lesion revascularisation), stent thrombosis, stroke, repeat revascularisation, rehospitalisation and bleeding events
- Additional secondary endpoints included contrast induced acute kidney injury, procedural safety outcome and angiographic or imaging based device success.
What is the main result?
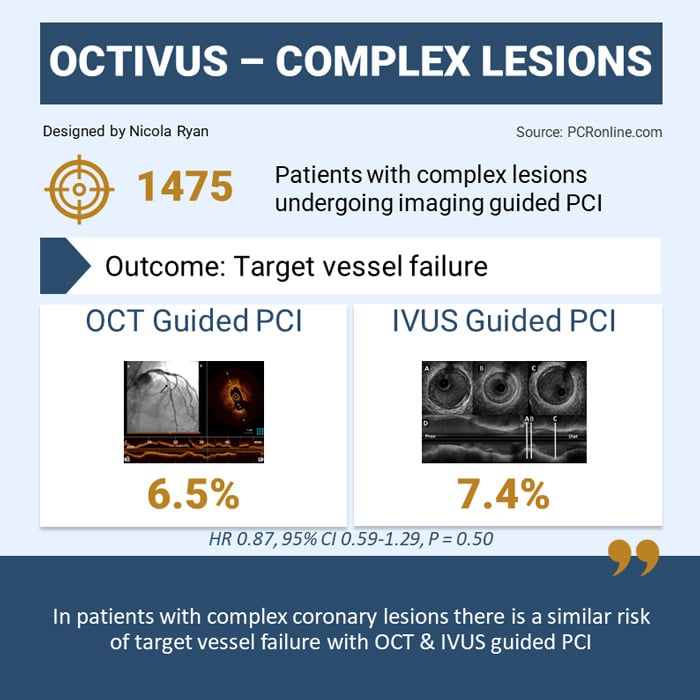
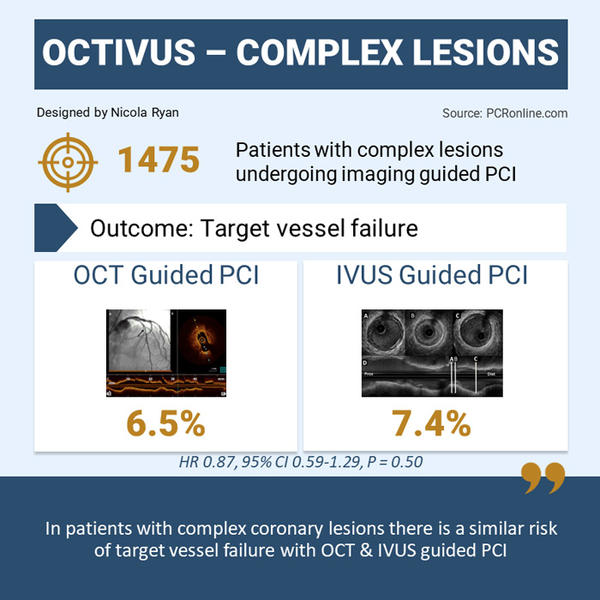
Overall, 2,008 patients were included in the OCTIVUS trial, of whom 1,475 (73.5 %) had complex coronary lesions, 719 (48.7 %) underwent OCT-guided PCI, and 756 (51.3 %) underwent IVUS-guided PCI.
Rates of prior PCI were slightly higher in the OCT group (25.7 %) compared to the IVUS group (21.0 %) with the remainder of the clinical characteristics well balanced between groups. From an anatomical and procedural point of view, the IVUS group had more LMS PCI, a higher SYNTAX Score and higher rates of imaging defined PCI success. Contrast use was higher in the OCT group with shorter procedural times and a lower complication rate compared to the IVUS group.
- There was no difference in TVF between groups 6.5 % OCT-guided vs 7.4 % IVUS-guided, HR 0.87, 95 % CI 0.59-1.29, p = 0.50
- Target vessel MI was higher in the IVUS group (0.8 % vs 2.4 %, HR 0.35, 95 % CI 0.14-0.88, p = 0.03)
- Target lesion (3.5 % vs. 4.4 %, HR 0.81, 95 %CI 0.48-1.36, p = 0.43) and target vessel (4.2 % vs. 4.9 %, HR 0.86, 95 %CI 0.53-1.40, p = 0.55) revascularisation were similar between modalities
- Contrast induced nephropathy rates were numerically higher, but did not reach statistical significance in the OCT group (1.9 % vs 1.5 %, HR 1..34, 95 %CI 0.61-2.93, p = 0.46)
Critical reading and the relevance for clinical practice
PICOT analysis of OCTIVUS study. Designed by Nicola Ryan
The results of this subgroup analysis of the OCTIVUS study show that, at 2-years follow-up, in patients with complex coronary lesions undergoing PCI, similar outcomes in terms of target vessel failure and target lesion failure are seen with OCT and IVUS guidance.
Rates of target vessel MI are lower with OCT guidance compared to IVUS guidance. It was hypothesised that the use vessel size parameters in the IVUS group lead to larger stent areas and larger maximum balloon sizing compared to the OCT arm, where whilst use of EEL measurements were recommended a lumen based approach was commonly used. This more aggressive stent sizing may have contributed to the increased rates of MI and in particular periprocedural MI in the IVUS group.
When anatomic complexity was analysed by subgroup, there were reduced event rates in patients with in-stent restenotic lesions with OCT guidance compared to IVUS (10.5 % vs 29.5 %, HR 0.36, 95 % CI 0.17-0.78). In all other lesion subtypes, there was no difference between imaging modality. Though events were overall small in the instent restenosis group and the outcomes may be due to play of chance the higher resolution of OCT allows more detailed characterisation of stent failure and may contribute to improved outcomes in this subgroup, by allowing operators to tailor treatment to failure mode.
Importantly, contrast use and the risk of contrast induced nephropathy is often seen as a limitation in the use of OCT. Whilst an increased contrast volume was used in the OCT arm, this did not translate into increased rates of contrast induced nephropathy. Patients with severe renal impairment were excluded from this study and whilst IVUS remains a preferable strategy in these patients, in the general population, the increased contrast use associated with OCT does not appear to translate into adverse outcomes. Though no complications were directly related to the use of imaging there was an increased incidence of procedural complications in the IVUS group (1.7 % vs. 3.4 %, p = 0.03). The authors hypothesised that the more aggressive stent sizing with IVUS guidance contributed to the increased procedural complications.
As with any analysis, there is a number of limitations which must be borne in mind, importantly overall event rates were low within the trial, therefore the ability to detect clinically and statistically relevant differences between subgroups is impeded. PCI optimisation requires not only the use of intravascular imaging, but appropriate interpretation and further treatment based on the results in this study imaging criteria for optimal stent implantation were achieved in < 50 % of cases. Therefore, it would be of interest to understand the differences in outcomes amongst patients who met imaging criteria for optimal stent implantation.
This study adds to the literature with regard to imaging guided PCI in complex coronary lesions. Whilst the inherent limitations of sub-group analysis stand, it would appear that in complex coronary lesions use of either form of intravascular imaging is appropriate and operators should focus on interpreting and appropriately reacting to the imaging findings.
Reference
- Kang DY, Ahn JM, Yun SC, Hur SH, Cho YK, Lee CH, et al. Optical Coherence Tomography–Guided or Intravascular Ultrasound–Guided Percutaneous Coronary Intervention: The OCTIVUS Randomized Clinical Trial. Circulation. 2023 Oct 17;148(16):1195–206.