COVID-19: Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine
COVID-19 vaccine Phase 3 clinical trials – what to look for?
- How is vaccine efficacy determined? - It is based on the ratio of confirmed COVID-19 infections in patients receiving a vaccine over those in the control arm.
- How long is the protective period? – The duration of follow-up after vaccination is important for assessing both the longevity of the effect of a vaccine, as well as its long-term safety profile.
- How is vaccine safety assessed? – It should be differentiated between a primary reaction to a vaccine, such as a skin reaction or post-vaccination fever (reactogenicity) and serious adverse events with potentially a lasting health impact.
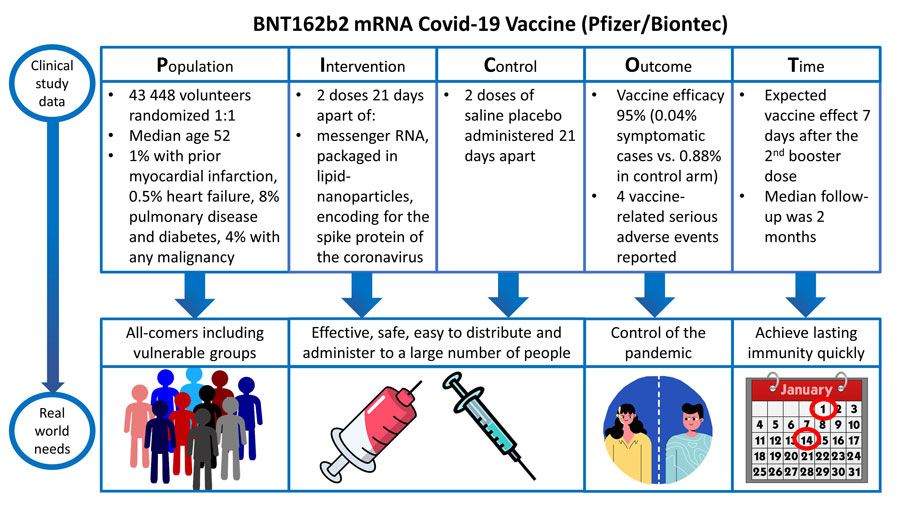
Clinical data and real-world demands of COVID-19 vaccines: BNT162b2 mRNA
Published in the New England Journal of Medicine on December 10th, 2020 (https://www.nejm.org/doi/full/10.1056/NEJMoa2034577)Quite unlike normal times, the majority of us (clinicians and public alike) first heard about the results of novel COVID-19 vaccine trials via press release and mainstream media outlets. Although this method of releasing trial data (ahead of peer reviewed publication) is unconventional, it must be acknowledged that we currently live in very unconventional times.Following the initial global celebration of the results of COVID-19 vaccine trials, it is essential (perhaps even more so) that we apply the highest level of critical appraisal of the published data as soon as it is released. To aid in this task we adopt the PICOT template to the critical appraisal of the BNT162b2 mRNA COVID-19 Vaccine (BioNTech and Pfizer).
POPULATION:
Patients of interest were adults 16 years of age or older who were either healthy or had stable chronic medical conditions (including but not limited to human immunodeficiency virus (HIV), hepatitis B virus, or hepatitis C virus infection).Key exclusion criteria included a medical history of COVID-19, treatment with immunosuppressive therapy, or diagnosis with an immunocompromising condition.Patients were recruited at 152 sites worldwide. A total of 43,548 participants underwent randomization, of whom 43,448 received injections. Key demographic features (of interest) included an almost equal split between males (51.1%) and females. The majority of patients were white (82.9%). Median age was 52 [16-89] years and 34.8% were categorised as clinically obese (BMI >30).
INTERVENTION:
The intervention of interest was the BNT162b2 mRNA COVID-19 vaccine: a lipid nanoparticle–formulated, nucleoside-modified RNA (modRNA) encoding the SARS-CoV-2 full-length spike. Participants in the vaccine group received two doses of 30 μg of BNT162b2 (0.3 ml volume per dose), 21 days apart, delivered in the deltoid muscle.
CONTROL:
The comparison of interest (control) group was a placebo (saline) injection. Study participants were randomly assigned (1:1) to BNT162b2 mRNA COVID-19 vaccine or placebo control.
OUTCOMES:
This trial reported both safety and efficacy endpoints. Interestingly, the authors present the safety data ahead of the efficacy data (perhaps designed to ensure a positive message of safety to the public).
Safety:
The primary safety outcome was those patients that solicited specific local or systemic adverse events and use of antipyretic or pain medication within 7 days after the receipt of each dose of vaccine or placebo. Furthermore, unsolicited adverse events through 1 month after the second dose and unsolicited serious adverse events through 6 months after the second dose were also recorded. Reactogenicity associated with the vaccine was generally mild or moderate and resolved within a couple of days. The incidence of serious adverse events was similar in the vaccine and placebo groups (0.6% and 0.5%, respectively). No deaths were considered by the investigators to be related to the vaccine or placebo.
Efficacy:
The primary efficacy outcome was of BNT162b2 against confirmed COVID-19 with onset at least 7 days after the second dose in participants who did not have serologic or virologic evidence of SARS-CoV-2 infection up to 7 days after the second dose. Among 36,523 participants who had no evidence of existing or prior SARS-CoV-2 infection, 8 cases of COVID-19 with onset at least 7 days after the second dose were observed among vaccine recipients and 162 among placebo recipients. This case split corresponds to 95.0% vaccine efficacy (95% confidence interval [CI], 90.3 to 97.6).
TIME:
Infection rates are measured 7 days after the 2nd dose as this is considered the start of the protective period. Because the COVID-19 vaccine trials are required to report their results as soon as possible, 2 months of follow up after the second dose of vaccine are available for half the trial participants and up to 14 weeks’ maximum follow-up for a smaller subset. The trial was originally designed for 2 year follow up, however, in view of the highly favourable results, placebo recipients will now be offered active immunization.
Strengths – Weaknesses – Opportunities – Threats (SWOT) analysis
The WHO has suggested that a minimum criteria for any vaccine against COVID-19 should be a “clear demonstration of efficacy with a ~ 50% point estimate”(1) which can be assessed by disease, severe disease or transmission endpoints. This analysis asseses the strengths and weaknesses and real-world applicability of the BNT162b2 mRNA Covid-19 Vaccine.
Strengths
- A large population of 43,448 participants, age range 16–89 years - including healthy people or people with stable chronic medical conditions, or immunodeficiency virus (HIV), hepatitis B virus, or hepatitis C virus infection.
- 42% of the enrolled population were above 55 years of age.
- The final analysis uses a success boundary of 98.6% for probability of vaccine efficacy greater than 30% to compensate for the interim analysis and to control the overall type 1 error rate at 2.5% - this led to a 99.9% certainty that the observed vaccine efficacy was >30%.
- Favourable overall safety profile, with the most commonly reported systemic events being fatigue and headache (59% and 52%, respectively, after the second dose, although fatigue and headache were also often reported in the control group (23% and 24%, respectively).
- The very low incidence of serious adverse events was similar in the vaccine and placebo groups (0.6% and 0.5%, respectively).
- Only 4 vaccine-related serious adverse events were reported in the BNT162b2 group (shoulder injury related to vaccine administration, right axillary lymphadenopathy, paroxysmal ventricular arrhythmia, and right leg paraesthesia).
- No deaths were considered by the investigators to be related to the vaccine or placebo. No Covid-19–associated deaths were observed.
- Between the first dose and the second dose, 39 cases in the BNT162b2 group and 82 cases in the placebo group were observed, resulting in a vaccine efficacy of 52% (95% CI, 29.5 to 68.4) during this interval and indicating early protection by the vaccine, starting after the 1st dose.
- The vaccine efficacy among subgroups defined by age, sex, race, ethnicity, obesity, and presence of a coexisting condition was generally consistent with that observed in the overall population.
Weaknesses
- With approximately 19,000 participants per group in the subset of participants with a median follow-up time of 2 months after the second dose, the study has more than 83% probability of detecting at least one adverse event, if the true incidence is 0.01%, but it is not large enough to detect less common adverse events reliably.
- This report includes 2 months and up to 14 weeks’ maximum follow-up for a smaller subset after the second dose of vaccine for half the trial participants: the occurrence of adverse events more than 2 to 3.5 months after the second dose and more information on the duration of protection remain to be determined.
- Although the study was designed to follow participants for safety and efficacy for 2 years after the second dose, given the high vaccine efficacy, ethical and practical barriers prevent following placebo recipients for 2 years without offering active immunization, once the vaccine is approved by regulators and recommended by public health authorities.
Opportunities
- In the context of the still expanding pandemic, the results of the BNT162b2 vaccine presented here suggest that it could, together with other public health measures, contribute to reducing the devastating loss of health, life, and economic and social well-being.
- This rigorous demonstration of safety and efficacy less than 11 months after the start of the pandemic provides evidence that mRNA-based vaccines could be a major new tool to combat pandemics.
- The continuous phase 1/2/3 trial design may provide a model to reduce the protracted development timelines that have delayed the availability of vaccines against other infectious diseases.
Threats
- To over-interpret these positive results to other vulnerable populations, such as younger adolescents, children, pregnant women or frail patients with multiple co-morbidities living in residential care settings.
- These data do not address whether vaccination prevents asymptomatic infection.
- Although the vaccine can be stored for up to 5 days at standard refrigerator temperatures once ready for use, very cold temperatures are required for shipping and longer storage.
- Long-term global safety for this new messenger RNA- based vaccine remains to be demonstrated.
Related links
- Safety and Efficacy of the mRNA-1273 SARSCoV-2 (Moderna) Covid-19 Vaccine
- Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2 (AstraZeneca/Oxford)
- Burning questions & real-world applicability
Authors
Date of publication: 17 December 2020






