COVID-19: safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine (Sputnik V)
Examination of the study methodology and results according to the PICOT principle, SWOT analysis and real-world applicability
- How is vaccine efficacy determined? - It is based on the ratio of confirmed COVID-19 infections in patients receiving a vaccine over those in the control arm.
- How long is the protective period? – The duration of follow-up after vaccination is important for assessing both the longevity of the effect of a vaccine, as well as its long-term safety profile.
- How is vaccine safety assessed? – It should be differentiated between a primary reaction to a vaccine, such as a skin reaction or post-vaccination fever (reactogenicity) and serious adverse events with potentially a lasting health impact.
An interim analysis of a randomised controlled phase 3 trial in Russia
Published in the Lancet on February 2nd, 2021 (https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32661-1/fulltext)
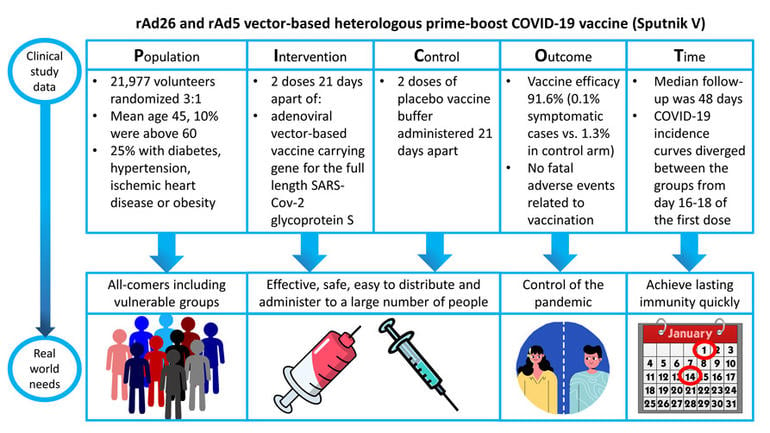
Examination of the study methodology and results according to the PICOT principle
Population of interest:
- 21,977 volunteers were randomized 3:1 to either the adenovirus-based vaccine (Gam-COVID-Vac) or a placebo
- 10% of the population were aged >60 years (38% female)
- 25% of the population had co-morbidities (DM, HTN, IHD, Obesity)
- Significant exclusion criteria included prior SARS-CoV-2, HIV, Hepatitis B/C, immunosuppression, or notably, acute coronary syndrome or stroke in the preceding year.
Intervention of interest:
- The vaccine consists of two vector components, rAd23-S and rAd5-S (two different recombinant adenoviruses), with the rationale of minimizing the risk of any pre-existing immunity to a specific adenovirus.
- The protocol mandates two deltoid intramuscular injections of rAd23-S (1011 viral particles, 0.5ml) on day 1 and rAd5-s (1011 viral particles, 0.5ml) on day 21
Comparison of interest:
Volunteers in the control arm received two deltoid intramuscular injections of the placebo vaccine buffer made up to equal the vaccine volume 21 days apart.
Outcomes of interest:
- 19,866 participants received both doses of the study medications (14,964 vaccine and 4902 placebo) and were included in the primary outcome analysis.
- The primary outcome was the proportion of participants with PCR-confirmed COVID-19 from day 21 after receiving the first dose.
- Covid-19 infection occurred in 16 participants in the vaccine arm (0.1%) and 62 participants in the placebo arm (1.3%) giving a vaccine efficacy of 91.6% (95% CI 85.6-95.2, p<0.0001)
- From a list of secondary outcomes, main findings appear to be a 100% protection from the severe form of COVID-19 conferred by the vaccine and the documented formation of spike protein-specific antibodies in 98% of the vaccinated participants. The secondary outcomes were severity of COVID-19; changes in antibody levels against SARS-CoV-2 glycoprotein S; proportion of participants with antibodies against SARS-CoV-2 N-protein; changes in SARS-CoV-2 neutralising antibody titres (increase of titres); changes in antigen-specific cellular immunity level (increase of cell-mediated immune response to antigen).
- Serious adverse events requiring hospitalization were assessed in all participants who received at least one dose, however, full data was not provided in this analysis, there were no fatal adverse events directly related to the vaccine.
- Rare adverse events were assessed in all participants who had received two doses, the majority of which were mild, i.e. Grade 1 (94%).
Time frame of interest:
Volunteers included in this analysis were followed for a median of 48 days (IQR 39-58) from dose 1 of vaccine.
Strengths – Weaknesses – Opportunities – Threats (SWOT) analysis
Strengths
- It is a stratified, double-blinded, placebo-controlled trial including SARS-COV-2 naïve participants:
- Stratified randomization was based on age, 18-30, 31-40, 41-50, 51-60, >60 years.
- SARS-CoV-2 PCR test was performed before each vaccine injection, which meant that asymptomatic cases could be documented and excluded at the time of the 2nd dose.
- The overall efficacy was high at 91.6% (95% CI 85.6-95.2, p<0.0001) and comparable to what was observed in the trials of messenger RNA vaccines. This overall result was consistent across all age groups.
- The safety of the rAd26 and rAd5 vector-based heterologous prime-boost Covid-19 vaccine was demonstrated with 94% of adverse events being mild (Grade I).
- Serious adverse event rates were low, 0.3% vaccine group, 0.4% placebo group, with none considered associated with the vaccine.
- Approximately a quarter of all participants had co-morbidities which potentially increased their risk of developing severe Covid-19 infection (diabetes, ischaemic heart disease, hypertension, obesity).
- No participants in the vaccine arm developed moderate or severe Covid-19 after the second dose of the vaccine
- Seroconversion was assessed in a small subgroup of the participants showing a seroconversion rate of 98.25% in the vaccine group and 14.91% in the placebo group
Weaknesses
- The majority of the included population were Caucasian (98.5%) and the trial was conducted entirely in Moscow, therefore efficacy may differ across ethnic groups.
- Only 10% of the overall population were over the age of 60, of this group the majority were between 61-69, and with less than 2% over the age of 80, which is an age range with increased morbidity and mortality associated with Covid-19 infection.
- Patients with asymptomatic Covid-19 PCR at time of second dose of vaccine were not counted as Covid-19 cases.
- Full data with regard to adverse events is not available in this interim analysis publication.
- A number of important subgroups at risk of increased complications from Covid-19 infection were excluded, including acute coronary syndrome and stroke in the preceding year and infection with hepatitis B, C or HIV.
- In this interim analysis, it has not been able to assess duration of protection; median follow-up time was 48 days after first dose.
- Unlike all hitherto published phase 3 clinical trials that utilized risk ratio as a measure of efficacy, vaccine efficacy was estimated based on the odds ratio in this study. Although the interpretation of odds ratio is less intuitive and more complex compared with risk ratio, it should be noted that at low frequency of events, as has been the case across all vaccine trials, the two measures are comparable.
Opportunities
- Although participants aged 18-30 had the highest titre, all other age groups, including those aged >60 had similar levels of humoral immune response. This is an encouraging finding in terms of maintaining vaccine efficacy in an elderly population who are prone to severe form of COVID-19.
- The vaccine studied in this trial was a liquid form which requires storage at -18°C, there is also a freeze-dried form which can be stored at 2-8°C which may allow easier distribution and storage worldwide.
- If the efficacy and safety data are confirmed when the final results of the phase 3 trial are published, this fourth vaccine (Gam-COVID-Vac) will provide an additional opportunity to vaccinate a larger number of populations worldwide.
Threats
- These are the results of an interim analysis, which was pre-specified after occurance of 78 events (confirmed symptomatic COVID-19 cases), therefore the final results of the phase 3 trial may change the conclusions drawn from this analysis.
- 2% of the sampled participants in the vaccine group did not develop spike protein receptor-specific antibodies. These vaccinated patients may have an increased risk of developing COVID-19 infection. On a research level, this could be a signal for post-marketing studies of this and other vaccines focusing on further exploring strategies to boost humoral response in such patients.
- The data was insufficient to assess asymptomatic or subclinical infection, with asymptomatic participants with positive Covid-19 PCR prior to their second vaccine dose not considered as Covid-19 cases. As this was the case across other hitherto published phase 3 trials as well, the effect of vaccines on asymptomatic virus spreading remains unknown.
- As with other vaccines, a longer-term follow-up may be needed to estimate the duration of the protective period with this vaccine.
- Pregnant women and children were excluded from this trial. For women of child-bearing potential, consent to use effective contraceptive methods and a negative urine pregnancy test were required.
- There were limited numbers of participants over the age of 60 and in particular over the age of 70 included in this trial. Interim analysis and the final results from this group in particular will be of interest.
- The large number of exclusion criteria must be taken into consideration before countries with different populations and health care regulations use the Gam-COVID-Vac vaccine.
Related links
- Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222)
- Safety and Efficacy of the mRNA-1273 SARSCoV-2 (Moderna) Covid-19 Vaccine
- Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine
- Burning questions & real-world applicability
Authors
Date of publication: 18 February 2021








