COVID-19: safety and efficacy of the mRNA-1273 SARSCoV-2 vaccine (Moderna) against SARS-CoV-2
Examination of the study methodology and results according to the PICOT principle, SWOT analysis and real-world applicability
- How is vaccine efficacy determined? - It is based on the ratio of confirmed COVID-19 infections in patients receiving a vaccine over those in the control arm.
- How long is the protective period? – The duration of follow-up after vaccination is important for assessing both the longevity of the effect of a vaccine, as well as its long-term safety profile.
- How is vaccine safety assessed? – It should be differentiated between a primary reaction to a vaccine, such as a skin reaction or post-vaccination fever (reactogenicity) and serious adverse events with potentially a lasting health impact.

Clinical data and real-world demands of COVID-19 vaccines: Moderna
Published in the New England Journal of Medicine30 December 2020 DOI: 10.1056/NEJMoa2035389
Published in the New England Journal of Medicine 30 December 2020 DOI: 10.1056/NEJMoa2035389 (https://www.nejm.org/doi/full/10.1056/NEJMoa2035389)
The Moderna mRNA-1273 SARS-CoV-2 vaccine is a lipid-nanoparticle (LNP)–encapsulated mRNA vaccine expressing the pre-fusion stabilized spike glycoprotein.
Here we summarize the analysis of the primary results of this ongoing pivotal phase 3 trial (COVE Trial).
Examination of the study methodology and results according to the PICOT principle
POPULATION:
- 30 420 volunteers were randomized 1:1 to either the mRNA-1273 vaccine or saline placebo.
- 25% of the population were 65 years or older (47% female)
- 17% of those below 65 years of age were deemed to be at risk of severe COVID-19 ( i.e. had at least one risk factor – chronic pulmonary disease, cardiac disease - mainly heart failure and pulmonary hypertension, diabetes, liver disease or HIV.)
- Cardiac disease (mainly heart failure) was present in 5% of the population
- Prior COVID-19 was an exclusion criterion however, 2.2% of the enrolled patients had evidence of Sars-Cov-2 infection by serology or PCR testing.
INTERVENTION:
- The vaccine consists of an mRNA sequence which encodes for the spike protein of the SARS-COV-2, encapsulated within a lipid nanoparticle.
- The vaccination protocol mandates two deltoid intramuscular injections of mRNA-1273 (100 μg) 28 days apart. More than 96% of participants received the second dose.
CONTROL:
- Patients in the control arm received two deltoid intramuscular injections of a saline placebo dose 28 days apart.
- Patients and medical staff observing the patients after vaccination, including outcome assessors, were blinded to the administered treatment. However, medical staff preparing and administering the vaccine were not blinded.
OUTCOMES:
- 28 207 participants received both doses of the study medication (14 134 vaccine and 14 073 placebo). These were included in the per-protocol efficacy analysis.
- The Primary end point was the efficacy of the mRNA-1273 vaccine in preventing symptomatic Covid-19 infection defined as at least two of the following symptoms: fever (temperature ≥38°C), chills, myalgia, headache, sore throat, or new olfactory or taste disorder, or as occurring in those who had at least one respiratory sign or symptom (including cough, shortness of breath, or clinical or radiographic evidence of pneumonia) and at least one test positive for SARS-CoV-2 by RT-PCR.
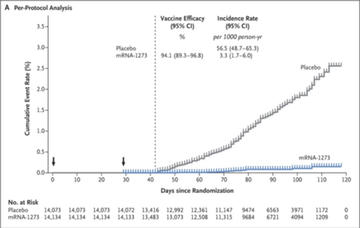
- Symptomatic COVID-19 infection occurred in 11 patients (0.08%) in the vaccine arm vs. 185 patients (1.3%) in the placebo arm. Based on these numbers, the efficacy of 94.1% was reported (calculated as 1 minus hazard ratio).
- The secondary efficacy endpoint was the occurrence of severe COVID-19: no severe COVID 19 cases were observed in the vaccinated population as compared with 30 cases (0.2%) in the control arm.
- The safety analysis: 30 351 participants received at least one dose of study medication and were thus included in the safety analysis (15 185 in the vaccine arm vs. 15 166 in the placebo arm). There were no fatal adverse events directly related to the vaccine.
TIME:
- Participants were followed for safety and the development of symptomatic COVID 19 over a median of 64 days (range 0-97) after the second dose.
Strengths – Weaknesses – Opportunities – Threats (SWOT) analysis
The WHO has suggested that a minimum criteria for any vaccine against COVID-19 should be a “clear demonstration of efficacy with a ~ 50% point estimate”(1) which can be assessed by disease, severe disease or transmission endpoints. This analysis asseses the strengths and weaknesses and real-world applicability of the Moderna vaccine.
Strengths
It is a stratified, observer-blinded, placebo-controlled trial including SARS-COV-2 naïve participants:
- Stratification was based on age and risk criteria for Covid-19 complications, i.e. being “at risk for severe illness”.
- The enrolment process was adjusted to increase the number of persons from racial and ethnic minorities.
- Participants were assessed for the presence of SARS-CoV-2–binding antibodies specific to the SARS- CoV-2 and a SARS-CoV-2 RT-PCR test was performed before each vaccine injection.
The primary outcome of the trial was reached
- The projected number of confirmed COVID-19 infections was 151 in order to have a statistical power of 90% to show at least a 60% vaccine efficacy:
- For the presented analysis, 196 cases of Covid-19 were confirmed, 11 cases in the vaccine group (3.3 per 1000 person-years; 95% CI, 1.7 to 6.0) and 185 cases in the placebo group (56.5 per 1000 person-years; 95% CI, 48.7 to 65.3), indicating 94.1% efficacy of the mRNA-1273 vaccine (95% CI, 89.3 to 96.8%; P<0.001).
- The vaccine efficacy to prevent Covid-19 was consistent across subgroups, in particular age above 65 years.
Weaknesses
- The trial Investigational New Drug sponsor, Moderna, was responsible for the overall trial design, site selection and monitoring, and data analysis.
- The short duration of follow up for safety and efficacy. This is of relevance since the length of the protection period conferred by vaccination is important both for individual health status and epidemiological planning.
- Although the planned number of cases exceeded what was statistically required for a power calculation, the fact that this happened during the pandemic surge in cases is a potential weakness of the trial. Per the trial’s statistical plan, an infection rate of 0.75% was expected, which would have amounted to 151 cases in 30 000 participants over a 6-month follow-up. The fact that 196 events accrued over a significantly shorter period of time (2 months) was due to a surge in cases in autumn of 2020 in the USA. This is inevitable in a pandemic, but the question remains whether the estimated vaccine efficacy that depends strongly on the rate of infection in the control group would be constant when the infection numbers in a population are at peak vs. nadir.
- No reliable correlate of protection (e.g. level of antibodies post vaccination) could be assessed due to very low numbers of COVID-19 cases in vaccinated participants (n=11).
Opportunities
- All 30 participants exhibiting severe Covid-19 symptoms were in the placebo group. This could suggest that mRNA-1273 is likely to have an effect on preventing severe illness, which is the major cause of health care utilization, complications, and death.
- Vaccine mRNA-1273 can be stored at 2° to 8°C (35.6° to 46.4°F) at clinical sites before preparation and vaccination. No dilution is required. Doses can remain at room temperature for up to 8 hours prior to administration.
- The encouraging results of this and a previous study of the BNT162b2 mRNA vaccine (Pfizer/Biontec) are important for proving efficacy and safety of the new concept of mRNA-based vaccines.
Threats
- The data was insufficient to assess asymptomatic or subclinical infection.
- The fact that the trial was conducted under the conditions of social distancing and other measures of disease prevention makes it questionable whether vaccine efficacy would be the same under the conditions of increased virus load without social distancing measures.
- Waning of efficacy over time has been demonstrated with other non Covid-19 vaccines. Therefore, a longer-term follow-up is needed to estimate the duration of the protective period with this vaccine.
- Pregnant women and children were excluded from this trial.
Related links
- Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine
- Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2 (AstraZeneca/Oxford)
- Burning questions & real-world applicability
Authors
Date of publication: 13 January 2021