Polymer-based versus polymer-free stents in high bleeding risk patients: final 2-year results from Onyx ONE
Selected in JACC: Cardiovascular Interventions by N. Ryan
The results of this study show that at 2-year follow-up HBR patients undergoing PCI with a polymer-based zotarolimus-eluting stent & 1-month DAPT had similar outcomes in terms of both safety & efficacy to those with a polymer-free biolimus A9-coated stent.
References
Authors
Stephan Windecker, Azeem Latib, Elvin Kedhi, Ajay J. Kirtane, David E. Kandzari, Roxana Mehran, Matthew J. Price, Alexandre Abizaid, Daniel I. Simon, Stephen G. Worthley, Azfar Zaman, Martin Hudec, Petra Poliacikova, Abdul Kahar bin Abdul Ghapar, Kamaraj Selvaraj, Ivo Petrov, Darren Mylotte, Eduardo Pinar, Raul Moreno, Franco Fabbiocchi, Sanjeevan Pasupati, Hyo-Soo Kim, Adel Aminian, Charles Tie, Adrian Wlodarczak, Seung-Ho Hur, Steven O. Marx, Ziad A. Ali, Maria Parke, Te-Hsin Lung, Gregg W. Stone, and on behalf of the Onyx ONE Investigators
Reference
J Am Coll Cardiol Intv. 2022 Jun, 15 (11) 1153–1163
Published
15 June 2022
Link
Read the abstract
Reviewer
Latest contributions
Balloon lithotripsy added to conventional preparation before stent implantation in severely calcified coronary lesions Impact of pullback pressure gradient on clinical outcomes after percutaneous coronary interventions Validation of intravascular ultrasound-defined optimal stent expansion criteria for favorable 1-year clinical outcomesMy Comment
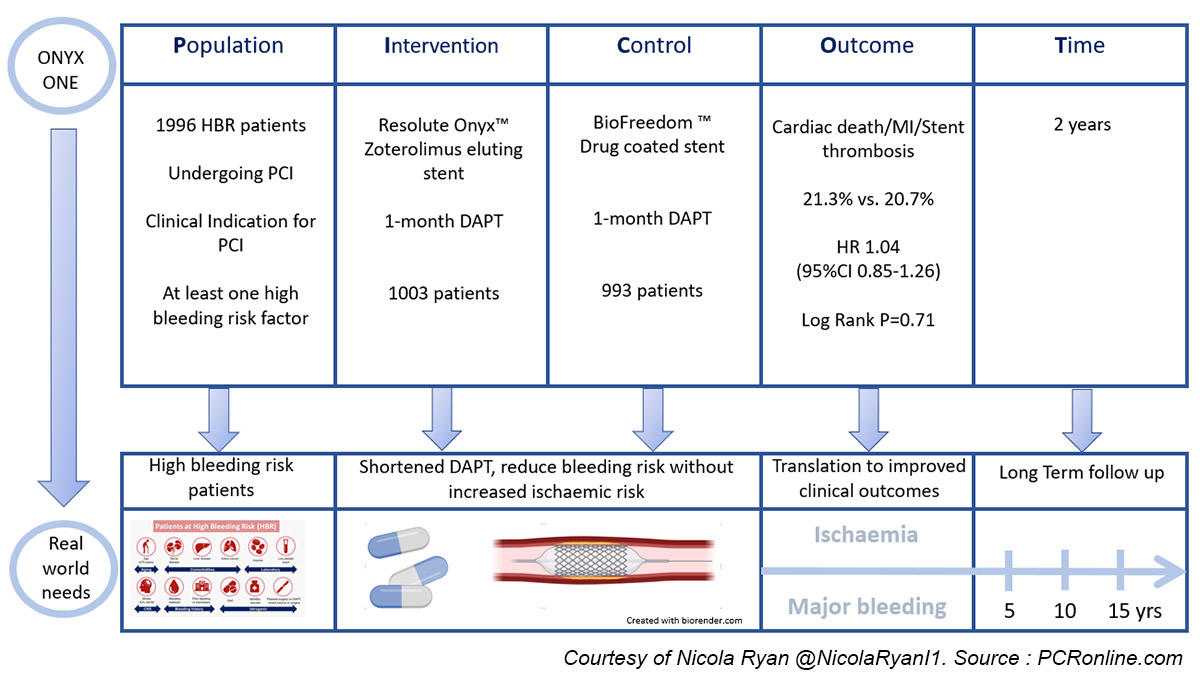
The Onyx One Trial is a prospective, randomised, multicentre, non-inferiority trial comparing the safety and efficacy of a polymer-based zotarolimus-eluting stent with a polymer-free biolimus A9-coated stent in high bleeding risk patients undergoing PCI treated with one month of DAPT followed by single antiplatelet therapy.
This paper presents the two-year outcomes of the study.
Why this study - the rationale / objective:
Drug eluting stents (DES) are currently the gold standard for patients undergoing PCI, with DAPT post PCI prescribed to reduce the ischaemic risk.
However, prolonged DAPT is associated with a concomitant increased risk of bleeding.
A significant proportion of patients undergoing PCI are at high bleeding risk, thus the excessive bleeding risk of extended DAPT in these patients may outweigh the ischaemic benefit.
Historically, HBR patients were treated with BMS’s however the LEADERS FREE trial demonstrated the superiority, both in terms of safety and efficacy, of a polymer-free biolimus A9 drug-coated stent in patients treated with one-month DAPT1.
The Onyx One Trial demonstrated the non-inferiority of a polymer-based zotarolimus-eluting stent to the polymer-free biolimus A9-coated stent in high bleeding risk patients treated with one-month DAPT at one year2.
In this paper, the authors report the two-year outcomes of the Onyx One Trial.
How was it executed? - the methodology:
Patients with coronary artery disease and a clinical indication for PCI, as well as at least one criteria for HBR, were randomised 1:1 to treatment with a polymer-based zotarolimus-eluting stent or a polymer-free biolimus A9-coated stent.
At the time of PCI, patients were prescribed DAPT, DAPT & OAC, or SAPT & OAC at the discretion of their treating physician for one month.
At one month, patients who remained event-free were prescribed SAPT (choice of agent at physicians discretion) or SAPT & OAC.
This analysis evaluated:
- the primary safety endpoint, the composite of cardiac death, MI, or definite or probable stent thrombosis at 2 years
- the secondary efficacy endpoint, target lesion failure (composite of cardiac death, target vessel MI or clinically driven target lesion revascularisation) at two years.
What is the main result?
Overall, 1,996 patients were included in the trial, 50 % presenting acute coronary syndrome, and a third were female with a high prevalence of traditional CVRFs.
The trial included a relatively older population with a mean age of 74 years, and a third of patients had concomitant atrial fibrillation.
At two years, 77 % of patients were prescribed SAPT (aspirin 65.1%, P2Y12 inhibitor 34.9%), 5.7 % were prescribed DAPT, and 16.7% OAC alone.
- There were similar rates of the primary safety endpoint, a composite of cardiac death, MI or definite or probable stent thrombosis in both groups at two years [21.2 % (ZES) vs. 20.7 % (DCS), Risk difference 0.5 %, 95 % CI -3.1 % - 4.2 %, p = 0.78]
- There was no difference in TLF between the groups at two years [22.1 % (ZES) vs. 20.9 % (DCS), Risk difference 2.0 %, 95 % CI -1.7 % - 5.6 %, p = 0.30]
- There was no difference in all bleeding [BARC 1-5; 22.9 % (ZES) vs. 20.7 % (DCS), Risk difference 0.5 %, 95 %CI -3.1 % - 4.2 %, p = 0.78 ] or major bleeding [BARC 3-5: 7.1 % (ZES) vs. 5.5 % (DCS), Risk difference 1.6 %, 95 % CI -0.5 % - 3.8 %, p = 0.16] between groups.
- Prespecified sub-group analysis including; ACS, age, gender, renal function, and OAC use did not demonstrate significant differences between the groups
PICOT Onyx One
Critical reading and the relevance for clinical practice:
The results of this study show that at two years follow-up, HBR patients undergoing PCI with a polymer-based zotarolimus-eluting stent and one month DAPT had similar outcomes in terms of both safety and efficacy to those undergoing PCI with a polymer-free biolimus A9-coated stent.
This is of interest as increasing life expectancy, as well as complexity of patients accepted for PCI, mean that HBR patients are commonly encountered in everyday clinical practice.
Importantly, 50 % of the population were patients presenting with ACS a group in which it may not always be possible to fully evaluate their bleeding risk prior to stent implantation.
Durable polymer DES are commonly used in clinical practice, therefore this evidence supporting short DAPT with a frequently used stent platform allows increased flexibility of stent choice, particularly in the setting of STEMI.
A higher all-cause mortality was seen in the ZES group, however, at multivariate analysis whilst COPD, DM, Prior MI, number of HBR criteria, occurrence of ST, CVA, spontaneous MI, and major bleeding were predictors of mortality at two years, stent type was not.
This finding is difficult to explain from the available data and requires further consideration moving forward.
Interestingly, a posthoc analysis showed that whilst the rates of both the primary safety and secondary efficacy endpoint were similar in both groups, from 31 days to 2 years, there was a higher rate of spontaneous MI’s in the DCS group (8.9 % vs. 6.2 %, p = 0.023). This is merely hypothesis-generating, however, along with the other data in this trial suggest that polymer-based ZES can be safely used in HBR patients with one-month DAPT.
It must be borne in mind that there is a number of limitations to this trial, including the fact that the trial was not powered for single endpoints such as death, MI, stent thrombosis, and repeat revascularisation, which would have required larger sample size.
Furthermore, physicians were at liberty to choose the single antiplatelet (aspirin or P2Y12 inhibitor) after one month; other studies have suggested that ticagrelor is preferable to aspirin as a SAPT after short DAPT though DAPT continued for three months rather than one in this trial3.
Whilst this study was not designed to support one month of DAPT in all patients, it does increase the evidence and confidence for physicians to abbreviate DAPT in carefully selected patients without any increased ischaemic risk.
As always, decisions with regard to ischaemic and bleeding risk need to be carefully assessed and individualised to each patient.
References
- Carrié D, Menown I, Oldroyd K, Copt S, Talwar S, Maillard L, et al. Safety and Efficacy of Polymer-Free Biolimus A9-Coated Versus Bare-Metal Stents in Orally Anticoagulated Patients: 2-Year Results of the LEADERS FREE Oral Anticoagulation Substudy. JACC Cardiovasc Interv. 2017 Aug 28;10(16):1633–42.
- Windecker S, Latib A, Kedhi E, Kirtane AJ, Kandzari DE, Mehran R, et al. Polymer-based or Polymer-free Stents in Patients at High Bleeding Risk. New England Journal of Medicine. 2020 Feb 12;0(0):null.
- Escaned J, Cao D, Baber U, Nicolas J, Sartori S, Zhang Z, et al. Ticagrelor monotherapy in patients at high bleeding risk undergoing percutaneous coronary intervention: TWILIGHT-HBR. European Heart Journal [Internet]. 2021 Oct 18 [cited 2021 Nov 2];(ehab702). Available from: https://doi.org/10.1093/eurheartj/ehab702






No comments yet!