30 Aug 2022
Durable polymer vs. biodegradable polymer DES in patients with ACS undergoing complex PCI: A post hoc analysis of the HOST-REDUCE-POLYTECH-ACS trial
Selected in EuroIntervention Journal by N. Ryan
The HOST-REDUCE-POLYTECH-ACS trial was a randomised, parallel-group multicentre trial with a 2x2 factorial design testing two independent hypotheses, one of which was the non-inferiority of durable polymer drug-eluting stents (DP-DES) to biodegradable polymer drug-eluting stents (BP-DES) in patients with ACS. In this post-hoc analysis, the authors evaluate the safety and efficacy of DP-DES and BP-DES in ACS patients undergoing complex PCI.
References
Authors
Doyeon Hwang, Hong-Seok Lim, Kyung Woo Park, Won-Yong Shin, Jeehoon Kang, Jung-Kyu Han, Han-Mo Yang, Hyun-Jae Kang, Bon-Kwon Koo, Yun-Kyeong Cho, Soon Jun Hong, Sanghyun Kim, Sang-Ho Jo, Yong Hoon Kim, Weon Kim, Sung Yun Lee, Seok Kyu Oh, Dong-Bin Kim, Hyo-Soo Kim
Reference
EuroIntervention. 2022 Aug 24;EIJ-D-22-00372
Published
August 2022
Link
Read the abstract
Reviewer
Latest contributions
Balloon lithotripsy added to conventional preparation before stent implantation in severely calcified coronary lesions Impact of pullback pressure gradient on clinical outcomes after percutaneous coronary interventions Validation of intravascular ultrasound-defined optimal stent expansion criteria for favorable 1-year clinical outcomesMy Comment
PICOT analysis of HOST-REDUCE-POLYTECH-ACS trial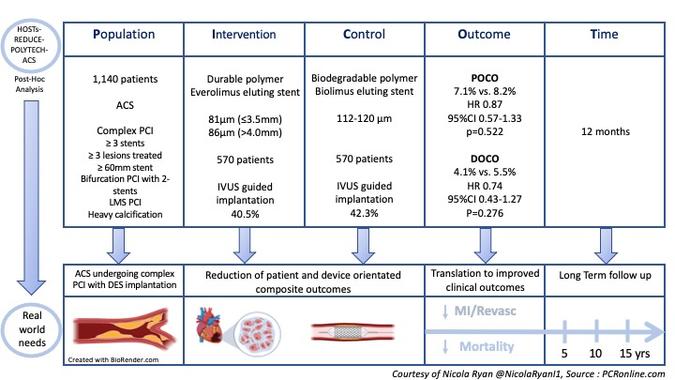
Courtesy of Nicola Ryan @NicolaRyan1, Source: PCRonline.com
Why this study – the rationale/objective?
Drug-eluting stents (DES) are currently the gold standard for patients undergoing percutaneous coronary intervention, however, there remains a not insignificant rate of adverse events, 2-3% per year. Advances in stent design, one of which is the use of biodegradable polymer, aim to reduce adverse events. A potential advantage of the biodegradable polymer is that after drug elution, the polymer degrades leaving behind a bare metal stent thus the local inflammatory reaction and consequent increased risk of stent thrombosis is reduced compared to a durable polymer. Patients undergoing complex PCI are at increased risk of adverse outcomes therefore BP-DES are potentially attractive in this group. To date, there is limited evidence comparing DP-DES and BP-DES in patients undergoing complex PCI.
How was it executed? - the methodology
Patients undergoing angiography acute coronary syndrome with one lesion >50% deemed to require treatment with DES were eligible for inclusion in the HOST-REDUCE-POLYTECH-ACS trial. Patients were randomised 1:1 to the DP-DES or BP-DES group and 1:1 to prasugrel 5mg or prasugrel 10mg. This post-hoc analysis included only patients undergoing complex PCI as defined by at least one of the following: ≥3 stents implanted, ≥3 lesions treated, bifurcation PCI with 2 stents, total stent length ≥60mm, left main PCI or heavy calcification. The use of intracoronary imaging was left to the discretion of the operator.
- The primary outcome was a patient-oriented composite outcome (POCO); a composite of all-cause death, non-fatal MI and any repeat revascularisation at 12 months.
- The secondary outcome was a device-orientated composite outcome (DOCO); a composite of cardiac death, target vessel MI and TLR
- Stent thrombosis, TVR and non-TVR were evaluated.
What is the main result?
Overall, between September 2014 and December 2018, 3413 patients were included in the trial, 1713 randomised to DP-DES and 1700 to BP-DES. Procedural data was available for 3301 patients of whom 1140 underwent complex PCI, 570 received DP-DES and 570 BP-DES. Of the complex PCI patients, three quarters of patients were men with a mean age of 64.6 years and high rates of common CVRFs. Stent length ≥60mm (59.2%) was the most common reason for PCI being deemed complex, with 19.6% and 13.9% undergoing bifurcation PCI with 2-stents and LMS PCI respectively. IVUS was used in 40% of complex PCI.
- The complex PCI group had an increased risk of both POCO (7.6% vs. 4.6%, HR 1.70, 95%CI 1.27-2.27, p<0.001, adj. HR 1.38, 95% CI 1.01-1.90, p=0.043) and DOCO (4.8% vs. 2.3%, HR 2.12, 95%CI 1.44-3.133, p<0.001, adj. HR 1.67, 95% CI 1.08-2.57, p=0.021) at 12 months compared to those not undergoing complex PCI.
- In patients undergoing complex PCI there were no significant differences in POCO between the stent groups (DP-DES 7.1% vs BP-DES 8.2%, HR 0.87, 95%CI 0.57-1.333, p=0.522).
- The rates of DOCE were similar between groups (DP-DES 4.1% vs BP-DES 5.5%, HR 0.74, 95%CI 0.43-1.27, p=0.278).
Critical reading and the relevance for clinical practice
The results of this post-hoc analysis show that in patients with ACS undergoing complex PCI, DP-DES and BP-DES had similar outcomes in terms of both patient and device-orientated clinical outcomes at one year. Compared to patients not undergoing complex PCI there was increased POCO and DOCO in patients undergoing complex PCI with no significant effects of the polymer type on clinical outcomes.
Multiple factors influence the outcomes after implantation of DES, including technical factors related to both implantation and stent design, clinical presentation, lesion characteristics and medical therapy. Disentangling the individual contributions of each component can be difficult and in this post-hoc, the authors attempted to assess the contribution of polymer design to outcomes in ACS patients undergoing complex PCI. This is of interest as ACS is a subgroup that may potentially benefit more from the biodegradable polymer design, particularly the potential reduced local inflammatory reaction and reduced risk of stent thrombosis compared to a durable polymer. Importantly at one-year follow-up, the polymer will not have been completely degraded therefore it will be of interest to see if a difference emerges between the groups at longer term follow-up.
Stent strut thickness is another aspect of the stent design which may contribute to adverse outcomes. In this study there was numerally lower DOCO in patients with DP-DES which the authors hypothesised may have been due to the thicker struts of the initial generation BP-DES however, a sensitivity analysis excluding thick struts did not show significant differences between the polymers. Furthermore, intracoronary imaging use may mitigate differences between stent designs however was only used in 40% of cases in this analysis. Importantly the CASTLE study which had prespecified use of OCT or IVUS in all patients did not show difference between DP-DES and BP-DES, furthermore, this population included both ACS and stable CAD (1).
As with any analysis, there are a number of limitations which must be borne in mind, most importantly the post-hoc nature of this study therefore the results can only be considered hypothesis generating. Improving clinical outcomes post complex PCI undoubtedly requires a combination of factors including use of appropriate imaging, plaque preparation, stent platform, stent optimisation and optimal medical therapy. This analysis supports the safety and efficacy of BP-DES in complex PCI in this population however longer term follow-up as well as randomised data is required to determine the optimal stent platform in complex PCI.
References
- Nakamura M, Kadota K, Nakagawa Y, Tanabe K, Ito Y, Amano T, et al. Ultrathin, Biodegradable-Polymer Sirolimus-Eluting Stent vs Thin, Durable-Polymer Everolimus-Eluting Stent. JACC Cardiovasc Interv. 2022 Jul 11;15(13):1324–34.







1 comment
J