13 Jun 2024
Intravascular ultrasound vs angiography-guided drug-coated balloon angioplasty: the ULTIMATE III trial
Selected in JACC: Cardiovascular Interventions by N. Ryan
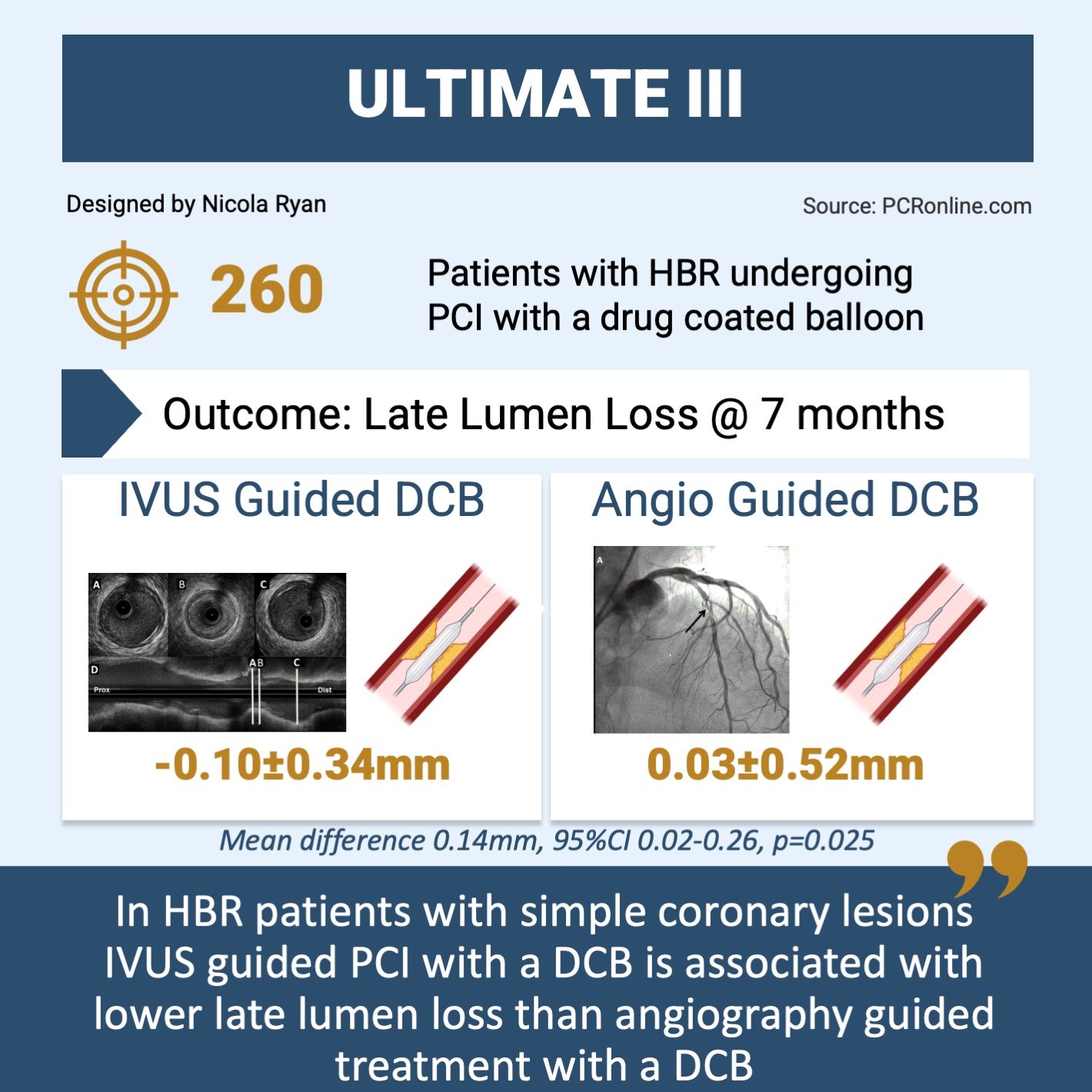
The results of this study show that IVUS guidance is associated with less late lumen loss compared to angiography guidance in HBR patients undergoing DCB angioplasty.
References
Authors
Xiao-Fei Gao, Zhen Ge, Xiang-Quan Kong, Xiang Chen, Leng Han, Xue-Song Qian, Guang-Feng Zuo, Zhi-Mei Wang, Juan Wang, Jia-Xian Song, Ling Lin, Tao Pan, Fei Ye, Yan Wang, Jun-Jie Zhang, Shao-Liang Chen, and the ULTIMATE Ⅲ Investigators
Reference
J Am Coll Cardiol Intv. Jun 05, 2024. Epublished DOI: 10.1016/j.jcin.2024.04.014
Published
Ahead of print - Jun 05, 2024
Link
Read the abstractReviewer
Latest contributions
Balloon lithotripsy added to conventional preparation before stent implantation in severely calcified coronary lesions Impact of pullback pressure gradient on clinical outcomes after percutaneous coronary interventions Validation of intravascular ultrasound-defined optimal stent expansion criteria for favorable 1-year clinical outcomesMy Comment
ULTIMATE III was a randomised control trial comparing intravascular ultrasound (IVUS) versus angiography guided treatment with drug coated balloons (DCB) in 260 high bleeding risk patients.
Overview of key data from the ULTIMATE III trial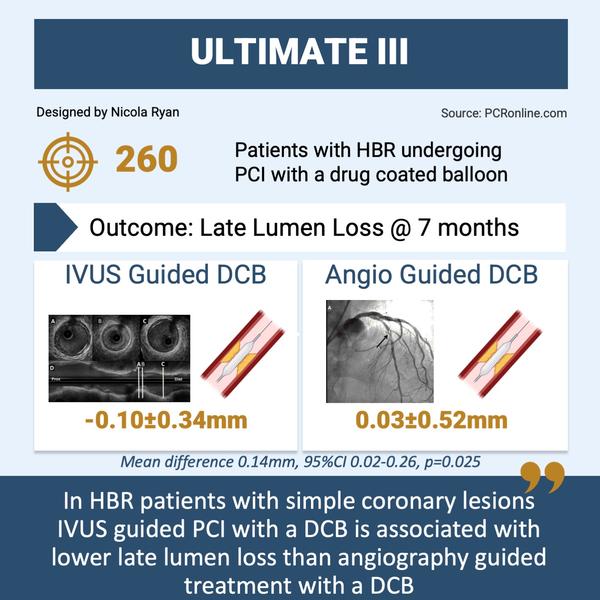
Why this study – the rationale/objective?
The benefits of IVUS guidance during implantation of drug eluting stents (DES) have been demonstrated; similarly, the use of DCB in de novo coronary lesions appears to be a safe and efficacious strategy in selected patients. However, the safety and efficacy of IVUS guidance in the treatment of de novo coronary lesions with DCB’s is not well established.
The ULTIMATE III trial aimed to assess the safety and efficacy of IVUS guided DCB angioplasty compared to angiography guided DCB angioplasty in HBR patients.
How was it executed – the methodology?
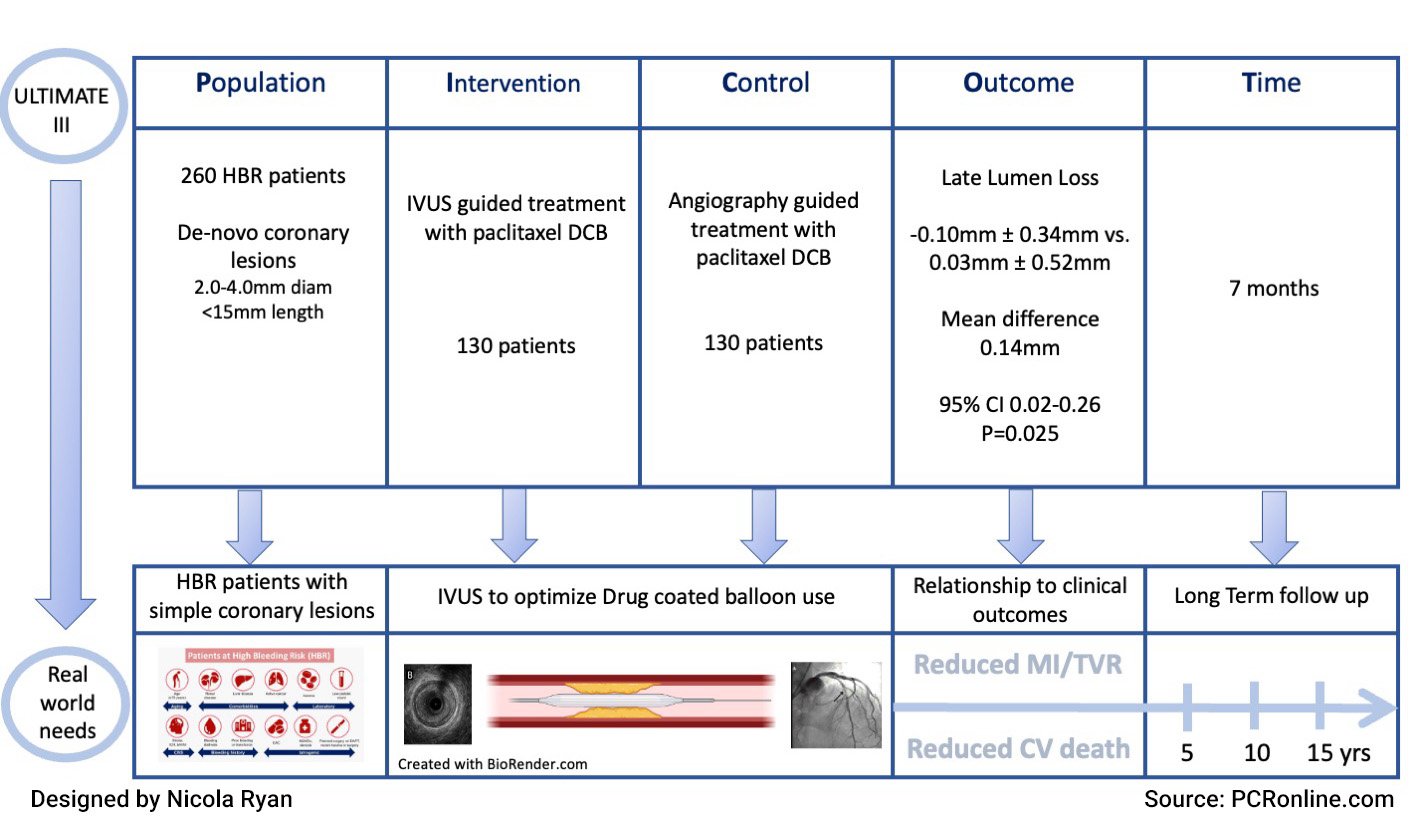
ULTIMATE III was a randomised control trial in HBR patients with silent ischaemia, stable or unstable angina, or MI > 48 hours post-presentation with de novo coronary lesions, undergoing PCI with DCB angioplasty. Coronary lesions were considered suitable for inclusion with a visual diameter of 2.0-4.0 mm and a lesion length < 15 mm. Patients were randomised in a 1:1 fashion to IVUS or Angiography guidance following initial angiography.
In the angiography guided group, the recommended balloon to distal vessel ratio was 0.8-1, based on visual assessment. In the IVUS guided group, the recommended balloon to distal reference diameter was 0.8 to media diameter or 1.0 to lumen diameter.
- The primary endpoint was late lumen loss (the difference between post-procedural minimal lumen diameter and follow up minimal lumen diameter) at 7 months.
- The major secondary endpoint was target vessel failure (TVF), a composite of cardiac death, target vessel MI, and ischaemia driven target vessel revascularisation at 6 months.
- Other secondary endpoints included acute coronary occlusion, spontaneous MI, ischaemia driven TVR, bleeding and the individual components of TVF.
What is the main result?
PICOT presentation of the ULTIMATE III study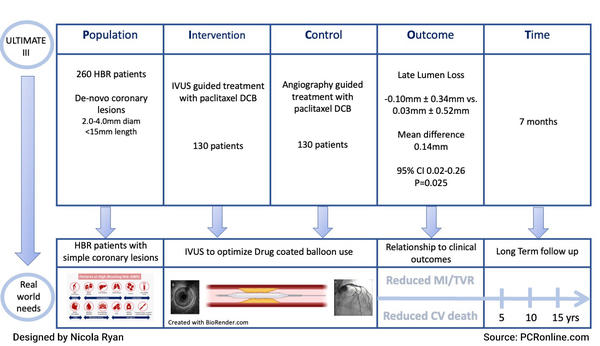
ULTIMATE III randomised 260 patients from February 2020 to December 2022, 130 to the IVUS guidance, and 130 to angiography guidance. 7-month follow-up angiography was available for 106 and 108 patients respectively. Almost 80 % of patients were male, with approximately three quarters presenting with unstable angina. Successful predilation was defined as ≤ 30 % residual stenosis, dissection ≤ type B and TIMI III flow. Following predilation, bailout stent was required in 2 patients in the angiography group, and 7 in the IVUS group (p = 0.172).
- At 7-month angiography follow-up, there was less late lumen loss in the IVUS guided group -0.10 mm ± 0.34 vs. 0.03 mm ± 0.52 (mean difference 0.14 mm, 95 %CI 0.02-0.26, p = 0.025).
- The IVUS group had a larger minimum lumen diameter (2.06 mm ± 0.62 vs. 1.75 mm ± 0.63, p < 0.001), and a lower diameter stenosis (28.15 % ± 13.88 vs. 35.83 % ± 17.69, p = 0.001) at angiographic follow-up.
- IVUS guidance led to the use of larger balloons for both predilation (2.73 ± 0.47 vs. 2.57 ± 0.50, p = 0.009) and DCB angioplasty (2.74 ± 0.46 vs. 2.56 ± 0.48, p = 0.005) despite similar baseline MLD and reference vessel diameter.
- There was numerically more TVF in the angiography group at six months, but this did not reach statistical significance (3.1 % vs 0.8 %, p = 0.37).
Critical reading and the relevance for clinical practice
The results of this study show that IVUS guidance is associated with less late lumen loss compared to angiography guidance in HBR patients undergoing DCB angioplasty. Despite similar MLD and reference vessel size, IVUS guidance led to use of balloons approximately 0.25 mm in diameter larger than angiography guidance alone. There was a non-significant increased rate of bail out stenting in the IVUS group 5, p = 0.46, with no penalty in terms of rates of final dissection (p = 0.46). Importantly, the study was not powered to detect differences in clinical outcomes between groups, and larger studies are required to determine if the angiographic benefits of IVUS guidance translate to improved clinical outcomes.
Optimal results with DCB’s require adequate lesion preparation prior to application of the DCB. IVUS assessment potentially enhances this by allowing precise measurement of the lumen diameters as well as evaluation of plaque volume and characteristics, lumen gain and dissections. To date, there have been no randomised control trials comparing IVUS to angiography guidance in DCB angioplasty, and no guidelines as to the optimal IVUS guided preparation/outcome in this population. This study provided recommendations for balloon sizing, however, it is not clear how suboptimal results were defined or treated, based on IVUS criteria. Importantly, IVUS assessment was not carried out at follow-up angiography ; therefore, it is not possible to determine mechanistically why the late lumen enlargement was greater in the IVUS group.
It must be noted that there are important limitations to this trial, including the fact that only relatively simple coronary lesions were included with severely calcified disease, multivessel disease and long lesions excluded. The included patients were a high bleeding risk population, therefore, the antiplatelet strategy was adjusted to this population and the results may not be generalisable.
In summary, ULTIMATE III provides data that IVUS guided DCB use leads to less luminal loss. Whether this angiographic endpoint translates into improved clinical outcomes requires evaluation in larger clinical trials. Furthermoren the optimal IVUS criteria to guide DCB use remains undetermined ; however, this trial increases the impetus to understand and develop the most appropriate criteria for IVUS guided DCB angioplasty.




1 comment
Until now, the axiom "the more you gain the more you lose" applied to angioplasty. These and other data show that the new axiom for angioplasty with DCB is: "the more you gain the more you get".